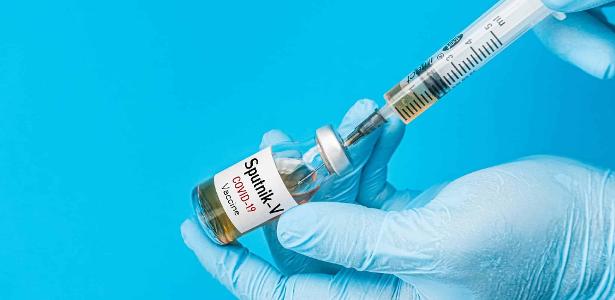
[ad_1]
The pharmaceutical company União Química arrived tonight with a request to Anvisa (National Health Surveillance Agency) for authorization to start testing phase 3 of the Sputnik V vaccine, which was developed for the first time in Russia. Anvisa now has 72 hours to give its opinion on the request.
Phase three consists of tests carried out with a group of human beings, in order to demonstrate the efficacy of the vaccine. At this point in the research, volunteers are vaccinated on a large scale, and the effects of the immunizing agent on the body are seen after the results.
This stage is the last phase of the clinical trial of a vaccine and one of the most important moments of research. The data obtained at this time must be submitted, published, and then the laboratory can enter the immunizer registration request.
If the União Química application is accepted by Anvisa, Sputnik V will be the fifth vaccine to receive authorization to start phase three of the tests. So far, the Oxford vaccine, CoronaVac, Pfizer vaccine and, more recently, the vaccine developed by Janssen have been approved by the Agency for studies at this stage.
Sputnik V began to be applied in Russia in early December. Today, Argentina has begun to vaccinate its population with the immunizer of Russian origin and Venezuela has announced an agreement to acquire doses of the vaccine.
Ministry of Health plans to start vaccination until February 10
At a press conference today at the Ministry of Health, the executive secretary, Elcio Franco, stated that he plans to start vaccination in Brazil no later than February 10. According to him, “in the best of cases”, the vaccines can begin to be applied on January 20.
According to the folder, the vaccination plan depends on the laboratories obtaining a definitive or emergency record of the vaccines produced. To date, no manufacturer of test immunizers in Brazil has requested regular or emergency use.
“It will depend on the logistics and the laboratories being up to date with Anvisa. It depends on the laboratory doing its part so that we are sure of the effectiveness and do not put the population at risk,” said the Secretary of Health Surveillance, Arnaldo Medeiros.
Brazil has the day with the most deaths registered since September
This was the second time in December that more than a thousand deaths were recorded in the country in a single day. On the 17th, the disease caused 1,054 deaths.
With State Agency