[ad_1]
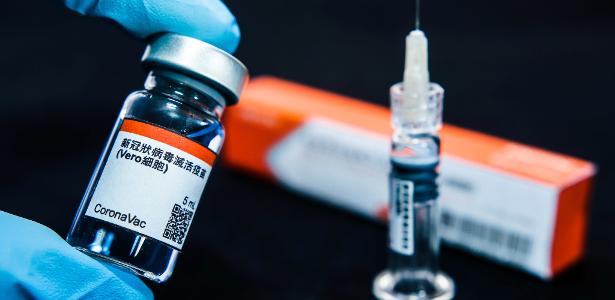
The Ministry of Health announced today that the federal government will purchase 46 million doses of CoronaVac, a coronavirus vaccine developed by the Butantan Institute, in São Paulo, in partnership with Chinese pharmaceutical company Sinovac Life Science.
THE Twitter found that the cost will be R $ 2.6 billion, considering the price in dollars of R $ 5.60, each dose will have a price of US $ 10.30 (around R $ 58). To this end, the ministry announced that a new MP (Provisional Measure) will be issued to grant a budget credit of R $ 1.9 billion.
The vaccine, according to Minister Eduardo Pazuello, will be included in the PNI (National Immunization Plan). “We have the experience of all the processes that involve this logistics, acquired during 47 years of PNI. The vaccines will reach Brazilians from all states,” said the general.
The announcement was made during a meeting with 24 governors this afternoon, in a virtual forum.
According to the Ministry of Health calendar, the 46 million doses are expected to be delivered in December 2020. Of these, six million will be produced in China and delivered in single-dose vials; the remaining 40 million, these in multidose vials, will be managed by the Butantan Institute.
The schedule also indicates that another 15 million multi-dose vials will be distributed by February 2021. In June 2021, another 40 million multi-dose vials are expected to be delivered, all produced by Butantan.
AstraZaneca will have production starting in April
In addition, the Fiocruz (Fundação Oswaldo Cruz) should start, from April, the own production of the AstraZeneca vaccine, developed with the University of Oxford (United Kingdom), and provide the country with up to 165 million doses during the second. 2021 semester.
As Butantan-Sinovac and AstraZeneca-Oxford are in advanced stages of production, both in phase 3, the last of the process, when tested on thousands of people, vaccination is expected to start in January 2021. But they have yet to be released by Anvisa (National Health Surveillance Agency) and have a guarantee of efficacy and safety, as determined by the Ministry of Health.
According to the folder, health professionals and risk groups should be the first to receive the vaccine.
The Ministry of Health also announced that it is monitoring more than 200 studies related to the production of vaccines against covid-19 and does not rule out new purchases, if necessary. The priority, according to the folder, is to deliver to the Brazilian population, in the shortest time possible, “a safe and effective solution to the disease.”
São Paulo does not provide forecast
After announcing that a possible vaccination of the São Paulo population against the coronavirus could begin this year, the government of João Doria took a step back and adopted a more cautious tone, saying that it is not yet possible to specify when the doses will be available.
“The prospects are optimistic, but we cannot give a precise date when this will happen. We hope that by the end of this year,” said the director of the Butantan Institute, Dimas Covas, yesterday during an interview at the Palácio dos Bandeirantes, seat of the government of São Paulo.
Previously, Doria had said that CoronaVac could begin to be applied to healthcare professionals as of December 15, if it passed all the tests. Covas, however, told the Twitter who “does not believe” in this period.
Experts have said that almost no vaccine is ready for use this year. The WHO itself (World Health Organization) declared that Brazil will not have a mass vaccination against the new coronavirus even next year. The organization believes that the world’s population will have to wait until 2022.