[ad_1]
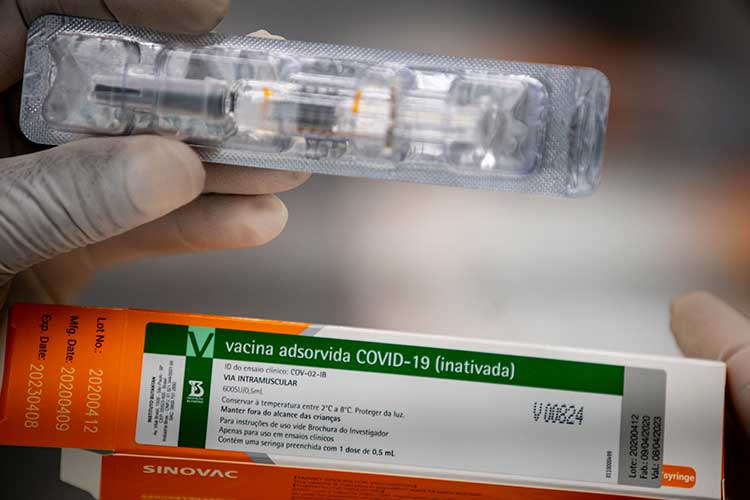
The team of the elected mayor Margarida Salomão (PT) estimates the commitment of R $ 53.5 million if the Municipality of Juiz de Fora actually buys one million doses of CoronaVac. As Margarida announced on December 18, the future municipal administration has already signed, with the Butantan Institute, a memorandum of understanding, that is, an agreement for the purchase of doses of the vaccine produced jointly between Butantan and the Chinese pharmaceutical company. Sinovac Biotech. The future Secretary of Health, Ana Pimentel, explained, this Wednesday (30), that the signing of the memorandum was a precaution taken so that Juiz de Fora had the vaccines available.
At this moment, as detailed Ana, the health team of the future management works with two scenarios while preparing the Municipal Immunization Plan against Covid-19 pending the regulation of vaccines by the National Health Surveillance Agency (Anvisa ). “The first is the one in which the federal government will assume the purchase of vaccines. In this scenario, the challenge for the Municipality is the organization of reception and vaccination, ”he said. “In the other scenario, we would be prepared, in budgetary, logistical and organizational terms, to acquire the doses of the vaccine. We are already diagnosing the entire structure we need to develop the Municipal Vaccination Plan. We are preparing according to the two scenarios ”.
READ MORE: Margarida Salomão announces inaugural actions of her government in PJF
Asked how long the Municipality would wait for a measure from the government of Jair Bolsonaro (without a party) to comply with the purchase of vaccines, Ana said that the cut-off line would be regulation and the possibility of marketing the immunizer. “What is outside our governance today is that vaccines must still be regulated by Anvisa, and then the Municipality can buy vaccines, which must also be regulated. If the federal government assumes its responsibility to distribute them, we will proceed through the National Immunization Program. As soon as it is possible, in legal terms, that the Municipality makes the purchase, we will do so, and precisely for that reason, we have already signed the memorandum of understanding ”.
As confirmed by the Butantan à Tribuna Institute, the estimated price of the CoronaVac dose is US $ 10.30, which, according to Wednesday’s dollar price, corresponds to approximately R $ 53.50. Therefore, the purchase of one million doses alone would lead the Municipality to spend around R $ 53.5 million on the immunizer alone. “We signed a memorandum of understanding for the acquisition of vaccines that foresees costs of around R $ 50 million. However, we will have other expenses and we are also already working with the budget and financial diagnoses to prepare. Today, for example, we have an installed capacity, in accordance with our previous immunization programs, to attend an average of 1,500 daily vaccinations. We will need to expand our capacity, not only using the public network itself, but also an accessory network ”.
Efficacy data is being evaluated by Sinovac
There were expectations that, last Wednesday (23), the Butantan Institute, together with the Government of the State of São Paulo, would disclose the CoronaVac effectiveness index after the conclusion of the final phase of clinical trials, which did not happen. . according to the institute, at the request of Sinovac himself, “contractually provided”. However, Butantan claimed that CoronaVac achieved an effectiveness rate higher than that recommended by the World Health Organization (WHO), that is, at least 50%.
In a note, Butantan noted that, last Wednesday, it sent Sinovac the primary database of the last phase of clinical trials carried out in Brazil. “The objective is to compare the data with the results of research in other countries, avoiding that the vaccine has different announced efficacy rates. The evaluation must be completed within 15 days. Then the final results will be sent to Anvisa and the National Administration of Medical Products of China ”.
On December 17, the Minister of the Federal Supreme Court (STF) Ricardo Lewandowski authorized states and municipalities to import and distribute vaccines against Covid-19 whose registration has been approved by the main international regulatory agencies if Anvisa does not approve it, within 72 hours, authorization requests received.
[ad_2]