[ad_1]
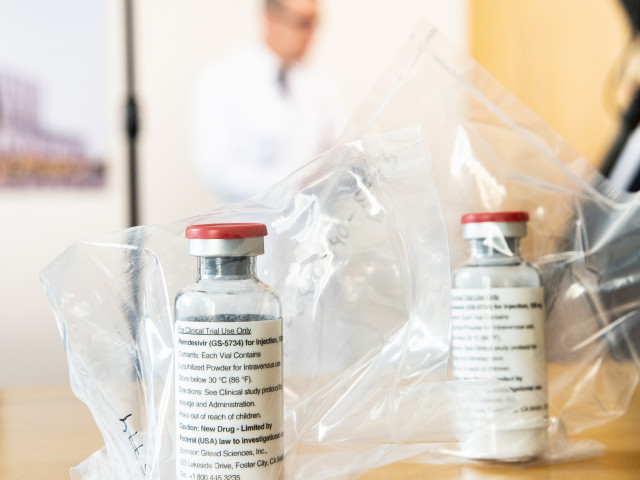
The United States Food and Drug Administration (FDA) has urgently authorized the use of the antiviral drug Remdesivir to treat patients with coronavirus, President Donald Trump said.
Remdesivir, produced by the pharmaceutical company Gilead Sciences, has been shown to be effective in facilitating the recovery of patients with coronavirus.
Donald Trump made the announcement at the White House, along with Gilead Sciences Director Daniel O’Day and Food and Drug Administration (FDA) Commissioner Stephen Hahn, according to Bloomberg and CNBC, according to Mediafax.
“We want to thank the staff who brought Remdesivir to this point and many of the people who were part of this effort, in fact, the medical staff,” said Daniel O’Day, noting that the company will donate a million doses of Remdesivir.
Immediately after the announcement, Gilead Sciences shares halted the downward trend, reaching $ 79.95 in New York. In extended trading, Gilead’s shares rose 2%.
Publisher: I. I.