
[ad_1]
According to the professor of the Clinic for Childhood Diseases of the Vilnius University Medical Faculty, the pediatrician of the Santara clinics prof. Vytautas Usonis, currently about 90 more vaccines are in the clinical research stages, 180 – in the preclinical research stages.
“I’m very hopeful that the vaccines that are available now are just the first few drinks and we will have many more. Today, a very important task is how to use them in the most effective way possible, “said the professor at an immunology conference held last Friday. V. Usonis.
The professor listed the stages of vaccine development:
1. Theoretical bases, knowledge of microbiology;
2. Vaccine development platform;
3. Preclinical studies;
4. Clinical trials consisting of 3 phases: the first phase involves dozens of volunteers, the second involves hundreds or thousands of volunteers, the third involves tens of thousands of volunteers;
5. Vaccine record;
6. Post-marketing surveillance of the vaccine, registry of adverse events.
According to the professor, vaccine development is not just a scientific job. It is also a huge bureaucracy, unimaginable volumes of documents, a huge amount of work.
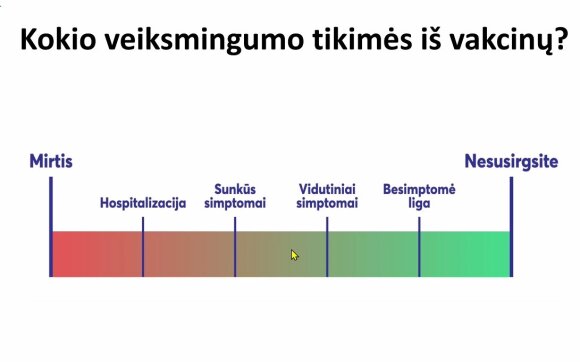
At the conference prof. V. Usonis also spoke about the fact that most people do not understand how the efficacy of the vaccine is evaluated. In fact, efficiency calculations are much more complex than they appear.
Initially, when evaluating the effectiveness of a vaccine, it should be compared with a placebo or a vaccine of similar composition. The placebo can be saline or ascorbic acid, they should not cause any reaction. If there is a uniform distribution of adverse events in both the vaccine and placebo groups, the vaccine is effective at 0.
“However, when it comes to a particular person, there can’t be 95 percent. risk of disease: get sick or not. As a result, it is necessary to look at other circumstances, what percentage of risk there is in one place or another, at one time or another.

Teacher. skilled. Dr. Vytautas Usonis
For example, estimates vary greatly from month to month. Let’s remember last summer, when there were large concentrations of people, but there were no illnesses, and in December, the risk was completely different, ”said the doctor.
It is common for vaccines to be tested at multiple sites. The wider the geography, the better, as it is important to see if the local environment can affect the way the vaccine works. As the doctor said, even when the same drug is tried in different places, at the same time, in South Africa and Brazil, there are big differences.
It is also important, according to the professor, what we expect from vaccines. Of course, most people think that vaccinated people are no longer at risk for infections and diseases. That would be an ideal option. Still, even less exposure to the vaccine helps prevent a pandemic.
© Photo of the organizers
“If a person dies, the effectiveness of the vaccine is 0 percent. But there are other options. Yes, it is ideal not to get sick after vaccination, but it may not be bad at all. [nesirgti sunkia ligos forma]. We get data that vaccinated people are infected, but this is usually a short-term, low intensity transmission of the virus: the amount of virus released by such a person appears to be small. These are primary data. Symptoms can be mild. More emphasis should be placed on the number of deaths among vaccinated people, “said Prof V. Usonis.
Huge resources have been used to develop vaccines.
The development of vaccines, according to the professor, involves a lot of technical and administrative work and many places where a lot of time could be saved. Still, time cannot be saved in biological terms.
“If the second dose of the vaccine is to be given after 3-4 weeks, it should not be given after a week or two. I am very pleased that there has been a discussion about extending the intervals between the first and second doses of RNA vaccines. It would be very prudent for that discussion to end altogether. It is not a race to be faster and vaccinated in a single dose. You can’t save time on vaccine safety, “said the doctor.
As the professor said, routine vaccine development often requires waiting for labs to release, which takes a long time to organize the data. For example, 44,000 studies have been conducted with the Pfizer vaccine.
“Imagine the flow of information, everything must be brought to the computer media, resources, people, time are needed,” said the professor.
At that time, the development of COVID-19 vaccines mobilized enormous resources that accelerated the entire process.
“We have said many times that it was ‘the first time’ with COVID-19 vaccines. In fact, the viral genome was identified so quickly for the first time. For the first time so many resources have been mobilized – intelligence, finance, technology and everything. others- to have the vaccines as quickly as possible, but in no way breaking the rules, so that speed is not at the expense of quality.
After all, adverse events must be controlled. There are huge databases in which such phenomena are recorded. However, the record of any side effects in the system does not mean that they actually happened due to the vaccine. Let’s remember a beautiful saying in Latin: what happened after that did not necessarily happen because of that ”, recalled prof. V. Usonis.
It is strictly forbidden to use the information published by DELFI on other websites, in the media or elsewhere, or to distribute our material in any way without consent, and if consent has been obtained, it is necessary to indicate DELFI as the source.
[ad_2]