
[ad_1]
The Moderna vaccine has already been approved by the US, UK (UK), and Israel, but the EU has so far only approved the first Pfizer-BioNTech vaccine.
Amsterdam-based EMA held its first meeting to approve the Moderna vaccine on Monday, but data was lacking to draw a conclusion and the agency requested more information.
This vaccine is the second to be approved by the EEA in an EU emergency. Last month a vaccine developed by Pfizer and BioNTech was approved.
“The EEA has recommended a conditional marketing authorization for the Modern COVID-19 vaccine, which is intended to protect people over 18 years of age from coronavirus disease in 2019,” the Amsterdam-based agency said in a statement. .
Kamala harris
© Zuma Press / Scanpix
The final decision is now up to the European Commission. There is no doubt that it will be positive. It would be the second vaccine registered in the EU against Covid-19 after Biontech and Pfizer.
The United States has already allowed the population to be vaccinated with the Moderna vaccine before Christmas.
Unlike the Biontech and Pfizer vaccine, it does not require extremely cold temperatures, making it easy to transport and store.
As evidenced by clinical trials with 30.4 thousand. The efficacy of the vaccines in the Modern American company is 94.1% compared to the placebo group.
© Zuma Press / Scanpix
Last week, the EVA said the coronavirus vaccines developed by British company AstraZeneca and the University of Oxford, which were approved in the UK last Wednesday, are unlikely to be distributed in the European Union until next month. .
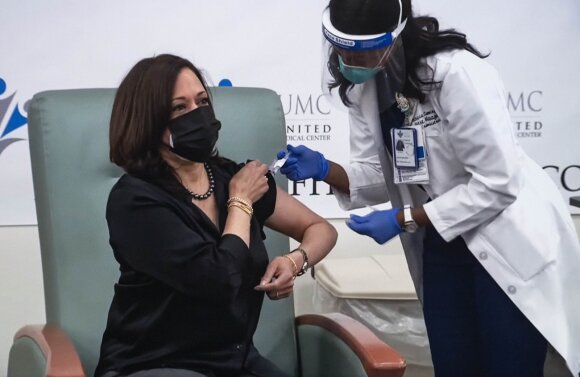
Kamala Harris, the president-elect of the United States, and her husband Dougu Emhoff have been vaccinated with the “Moderna” company.
“I want to encourage everyone to get vaccinated,” Harris, 56, said in late December. – It is directly related to saving lives. I trust the science and scientists who developed and validated this vaccine. “
Two doses of the vaccine are needed to develop immunity, the second is given later, after a break.
It is strictly forbidden to use the information published by DELFI on other websites, in the media or elsewhere, or to distribute our material in any way without consent, and if consent has been obtained, it is necessary to indicate DELFI as the source .
[ad_2]