[ad_1]
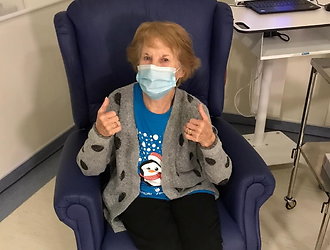
This important step came when the United States Food and Drug Administration (FDA) committee met to discuss a proposal to approve this COVID-19 vaccine.
An editorial relating to a scientific article on the clinical trial results of the Pfizer and BioNTech vaccines states: “The trial results are impressive enough to be presented as an example of any analysis imaginable. It is a triumph. “
Almost 44,000 people participated in the vaccine trials. volunteers: several thousand more than reported in previous analyzes. About half of them received the vaccine and the rest received the placebo.
The article confirms that after two doses of the BNT162b2 vaccine, 95% of the subjects acquired resistance to the coronavirus.
The vaccine had a similar effect on different ages, sex, race, nationality, body mass index [asmenis]as well as comorbidities, ”write the article’s authors.
During the trials, 10 cases of severe COVID-19 were reported in the participants after the first injection. Nine of these people had received placebo and one had received the vaccine.
An editorial accompanying the study mentions some “minor problems.”
“The number of severe COVID-19 cases (one in the vaccinated group and nine in the placebo group) is too small to draw any conclusions as to whether rare cases of infection in vaccinated people are in fact more severe,” the authors noted.
Another question is whether there may be some unidentified safety problem when millions or perhaps billions of people get vaccinated with the vaccine.
It is also unknown whether more side effects will occur during the follow-up of study participants. It is not yet clear how long the protective effect of the vaccine will last, if it will reduce the infectivity of the coronavirus or how it will affect children, pregnant women and patients with severely weakened immune systems.
[ad_2]