[ad_1]
Clinic and development in Korea 15 Corona 19 treatments and 6 vaccines
(Seoul = Yonhap News) Reporter Seung-Hyun Gye = The Ministry of Food and Drug Safety announced on the 31st that it approved phase 1 and 2 clinical trials of ‘GBP510’, a new vaccine against coronavirus infection (Corona 19 ) developed by SK Bioscience.
This clinical trial aims to evaluate the safety and immunogenicity of GBP510 in healthy adults. After clinical phase 1, phase 2 is performed sequentially.
As a result, the number of drugs currently being developed with approval for COVID-19-related clinical trials in Korea has increased to 15 treatments (13 components) and 6 vaccines.
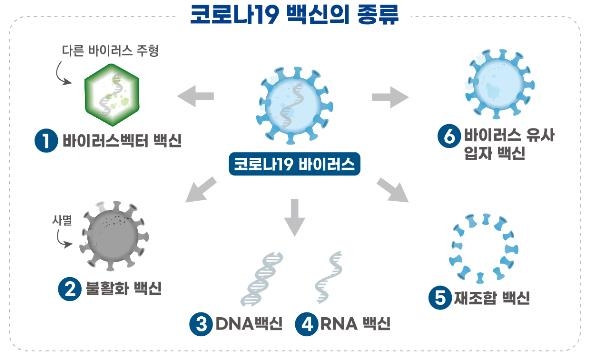
GBP510 is a “combination vaccine” that uses genetic recombination technology to produce the Corona 19 virus surface antigenic protein.
When the vaccine surface antigen protein stimulates immune cells, neutralizing antibodies are formed that induce an immune response. When the Corona 19 virus invades, the antibodies will kill the virus.

[식품의약품안전처 제공. 재판매 및 DB 금지]
The recombinant vaccine ‘NBP2001’, which was previously approved for the phase 1 clinical trial by SK Biosciences on the 23rd of last month, induces an immune response with the Corona virus surface antigen protein 19.
GBP510 differs in that surface antigen proteins induce an immune response by binding to specific proteins that form icosahedral nanostructures.
Overseas, NovaVax of the United States is conducting phase 3 clinical trials of a COVID-19 vaccine using genetic recombination technology.
[표] Types of Clinically Approved Vaccines in Korea
| < 국내 임상 승인된 백신 종류 > | ||
| Type of vaccine | product | Clinical stage |
| DNA vaccine (3 cases) | International Institute for Vaccine Research (INO-4800) | 1 2 phases |
| Genexine (GX-19N) | 1 2 phases | |
| Jinwon Life Sciences (GLS-5310) | 1 2 phases | |
| Recombinant vaccine (2 cases) | SK Bioscience (NBP2001) | 1 phase |
| SK Bioscience (GBP510) | 1 2 phases | |
| Virus vector vaccine (1 case) | Celed (AdCLD-CoV19) | 1 2 phases |
※ Provided by the Ministry of Food and Pharmaceutical Safety.
<저작권자 (c) 연합 뉴스,
Unauthorized reproduction-redistribution prohibited>
2020/12/31 19:13 Sent