[ad_1]
Entry 2020.10.20 07:15 | Revision 2020.10.20 07:20
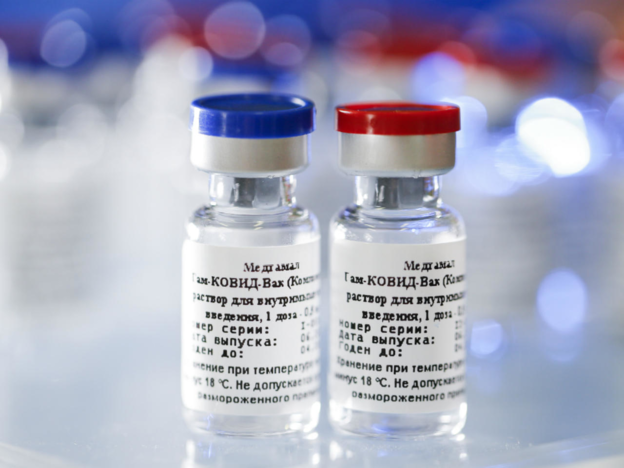
On the 19th (local time), according to Sputnik News, President Kirill Dmitryev of the Russian sovereign wealth fund (RDIF) held an online seminar on the topic of cooperation with the countries of South America. It is planned to produce ash, “he said,” it is possible to supply in bulk in December. ”
This is the first time that the Russians have announced the decision to produce Sputnik V in Korea. In an interview with the media last month, CEO Dmitryev said: “Negotiations on the production of Sputnik V in Korea are in the final stage.” The negotiations were reported to have taken place with large Korean pharmaceutical companies.
Dritriev said negotiations are also underway with foreign health authorities to obtain approval for the use of the vaccine. Mexico has already signed a vaccine supply agreement·Brazil·Argentina followed by India·It is also negotiating supply with Peru and plans to announce specific details soon.

However, unlike the usual vaccine development procedure, controversy over efficacy and safety arose when the phase 3 clinical trial was skipped. Russia plans to complete phase 3 trials by the end of this month and begin mass vaccinations. scale.
Western countries, like the United States, are showing doubts about vaccines made in Russia. They are reportedly engaged in “vaccine diplomacy” to increase their influence in the international community. In response, the Russian government published the results of the phase 1 and 2 tests in the international medical journal Lancet, refuting that “through two clinical trials conducted in June and July this year, antibodies were formed from all participants and there were no serious side effects. ” did.