[ad_1]
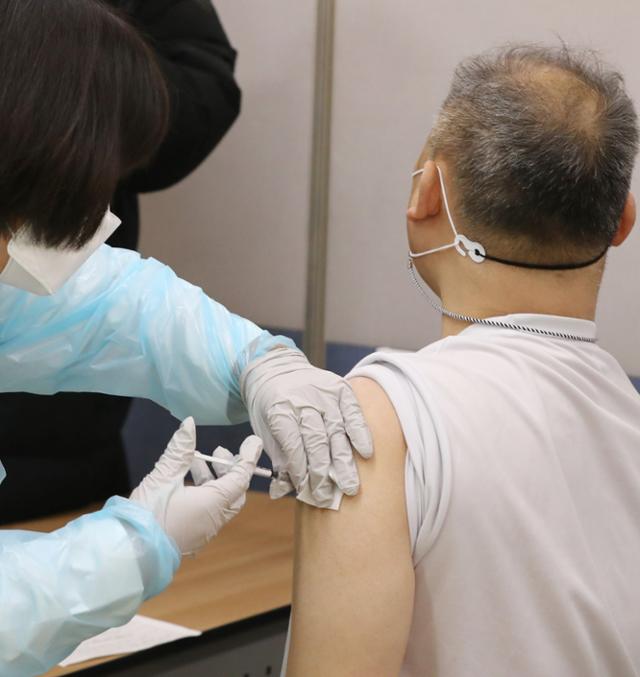
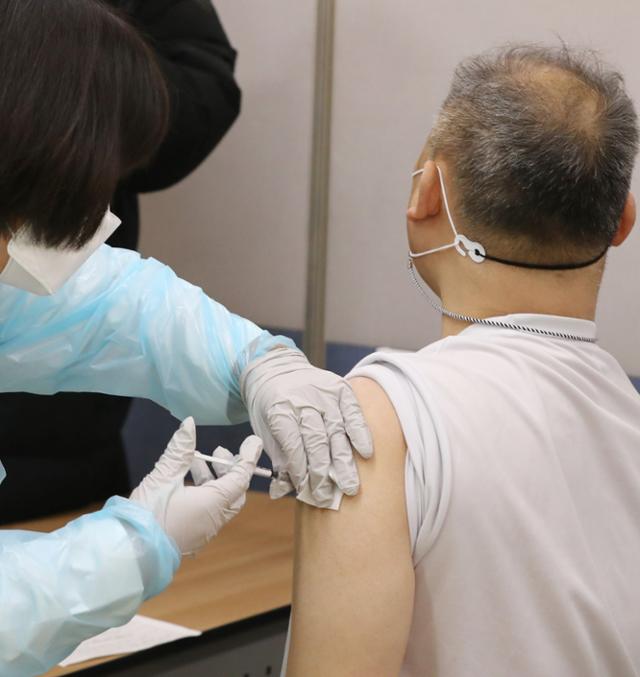
Citizens will receive the AstraZeneca (AZ) vaccine at the Hongseong Health Center in Chungnam on the morning of the 26th when vaccination against the new coronavirus infection (Corona 19) began. News 1
Kang-rip Kim Director of Food and Drug SafetyThis 26 “AstraZeneca (AZ) vaccine has been accredited in a triple consultation process by experts“I said.
In an interview with KBS Radio’s ‘The Strongest Preview of Management’, Director Kim said: “AstraZeneca products being inoculated as of today have been thoroughly verified for safety and efficacy through a national approval process.” “Edo (for actual vaccination) was recognized in the triple expert consultation procedure.”
“In the case of a triple consultation process, When new drugs arrive, the central pharmacist review committee listens to expert opinions, and a new professional verification system has recently been introduced at the working level staff level with this counseling system as a triple.“He emphasized.
He said: “Finally, it goes through the Central Pharmacy Review Committee, but finally, it goes through the final review committee, where external experts participate,” he said.
Director Kim said: “In the permitting process, we examined it as a document without experimentation, In the case of vaccines, a procedure to test the safety of the quality of the approved drug through experiments.“You go through this procedure,” he explained. “This AstraZeneca vaccine also went through this procedure.”
“You don’t have to worry about the safety of the AstraZeneca vaccine”

On the morning of the 26th, nursing home workers who received a new coronavirus (Corona 19) infection AstraZeneca Corona 19 at the Busanjin-gu Health Center in Busan are showing their vaccination confirmation after receiving the first dose of Corona 19 AstraZeneca vaccine. Yunhap news
Regarding efficacy for people 65 and older, “I look at two things when looking at vaccines. One is, is it safe, and then is it effective?”ThatHowever, when comparing the vaccines of the elderly over 65 with those of those under 65, the rate of adverse reactions is significantly lower, so safety concerns need not be concerned.“I explained.
Director Kim said, “However, there was a regret in the part of people 65 and older participating in the clinical trial, and due to a lack of numbers, it was a shame that we couldn’t find a statistically significant value to determine if the vaccine was effective. ”
He continued: “We are now targeting 10 million people in the United States, and 20% are 65 or older, so when we can confirm this result to some extent, we can make additional decisions in the areas that were insufficient. ”
Director Kim’s Pfizer vaccine is scheduled to be vaccinated on the 27In this regard, he introduced, “It is an unstable vaccine that requires cryogenic distribution,” he said. “We are freezing at very low temperatures, but we prepare and train them early with field experts.”
Then he said “The simulation training was also completed through the designation of a specialized company that can be engaged in cryogenic refrigeration distribution as a consignment company.“The Pfizer vaccine has gone through the second phase of the triple validation phase and we intend to finalize the license without going through next weekend.”
Son of sungwon reporter [email protected]
News directly edited by Hankook Ilbo can also be viewed on Naver.

Issues that may interest you
[ad_2]