
[ad_1]
Entry 2020.12.24 12:00 | Revision 2020.12.24 14:36
Blood diagnostic accuracy 30% … Fewer cancer-related substances in urine
High-sensitivity sensor improves detection performance and is precisely analyzed by AI

The Korea Institute of Science and Technology (KIST) announced on the 24th that the research team at Dr. Lee Kwan-hee’s Biomaterials Research Center has developed a new cancer diagnostic technology that combines ultra-sensitive biosensors and artificial intelligence ( IA) with the research team of Professor Jeong In-gap from Asan Seoul Medical Center.
Prostate cancer still has a blood test diagnostic technology, but the accuracy is only 30%. For a diagnosis close to 100%, a biopsy, an invasive method, is required. Urine also contains several types of prostate cancer biomarkers (substances in the body that determine whether the disease has occurred or not), but the amount was small, so it was not used for diagnosis.
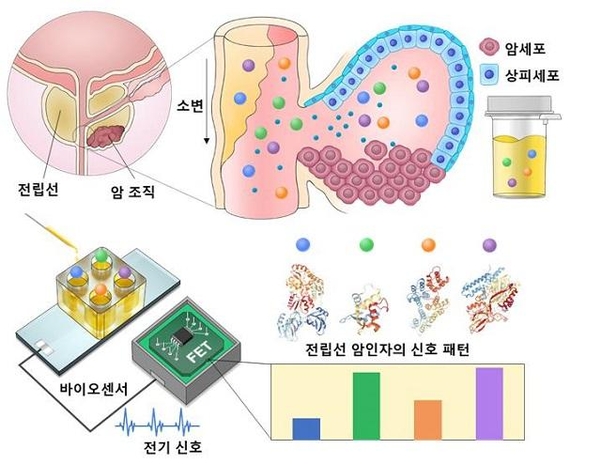
When the research team used this technology to analyze urine samples from 76 patients or the general public, it was able to distinguish between patients with 95.5% accuracy.
As a next step, the research team plans to conduct clinical trials with 500 subjects (patients or the general public) to further improve precision and commercialize them in 2022-2023.
Professor Jeong said: “If it is commercialized, it will be possible to drastically reduce medical expenses and the burden of medical personnel by minimizing unnecessary biopsies and treatments.” Dr. Lee hoped that “it could be used for an accurate diagnosis of other carcinomas.”
The results of the research were published in the latest issue of ACS Nano, an academic journal published by the American Chemical Society (ACS).