[ad_1]
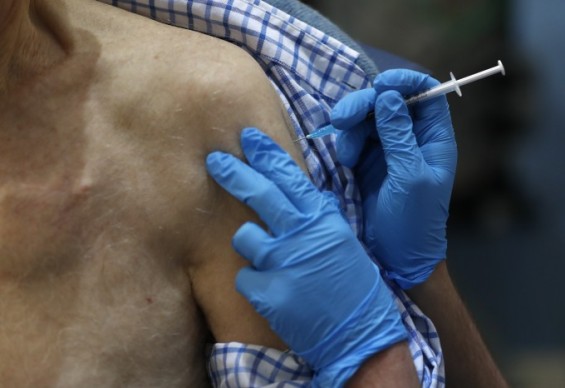
The vaccination takes place at a vaccine center in a London hospital on the 8th (local time), when the UK started the world’s first comprehensive vaccination against Pfizer’s new vaccine against coronavirus infection (Corona 19). Yonhap news provided
The world’s first vaccine against a new coronavirus infection (COVID-19, Corona 19) developed by the American pharmaceutical company Pfizer and the German Bioentech was launched in the United Kingdom on the 8th (local time). However, it was confirmed that two people had an allergic reaction on day 9 (local time), one day after onset. Consequently, the UK Medicines Regulatory Authority has issued a recommendation that people with severe allergic reactions to medicines or food should not receive the vaccine. Vaccines from the University of Oxford in the UK and AstraZeneca, which Korea signed a large-scale pre-purchase contract, are being questioned about the safety of “low doses” and the lack of data in clinical trials for older people. The United States Food and Drug Administration (FDA) is currently delaying approval of the vaccine.
The UK Sky News reported that two people who had been vaccinated by Pfizer had allergic reactions. They are known to have had an anaphylactic reaction, in which the body overreacts to certain substances after being vaccinated on day 8 as employees of the UK National Health Service (NHS). It is said that he is currently recovering.
○ Mixed reactions to allergy results … Lack of clinical data related to pregnant women and adolescents
The NHS and the Medicines and Healthcare Products Regulatory Agency (MHRA) announced that they were conducting an investigation and that those who had allergic reactions to medicines, foods and vaccines in the past should not receive the Corona 19 vaccine. “Allergic reactions are common in new vaccines and are preventive measures, “said Stephen Foyce, head of healthcare at NHS England. “The MHRA has been told that people with a history of allergic reactions should not get vaccinated.”
Amy Rose, a Pfizer spokeswoman, said: “The MHRA has provided interim guidelines to apply during the investigation to provide a full understanding of the cause of the allergic reaction.” “Pfizer and Bioentech will support the research.”
Anthony Pouch, director of the National Institute of Allergy and Infectious Diseases (NIAID), evaluated this as an “unusual and rare reaction.” In an interview with CNN on the 9th (local time), he said: “This is very likely an abnormal and rare reaction. If you have an allergy, you should be prepared to treat it because you may have this reaction.”
Pfizer also excluded people with a history of allergies such as anaphylactic reactions from the subjects due to concerns about side effects during the initial clinical trial. According to data submitted to the US Food and Drug Administration (FDA), 0.63% (137 patients) experienced hypersensitivity side effects such as facial nerve palsy in clinical trials, slightly higher than those taking placebo (0.51%, 111 patients). It was high. It is the result of excluding people with a history of allergies, so the related information is insufficient.
Stephen Evans, a professor of pharmacokinetics at the University of London’s University of Hygiene and Tropical, told the UK’s Science Media Center: “Few have allergic reactions, but the numbers are very uncertain.” I couldn’t imagine if I could see it. “However, he added,” this news does not mean that the general public should be concerned, “he added.” Vaccination can be stopped and investigated until the cause of the allergic reaction is found. ”
In addition to those with allergies, clinical data on pregnant women and adolescents are also sparse. Pfizer supports it too. Pfizer said that “currently, data related to vaccination for pregnant women is limited,” he said. “If you are pregnant, planning to become pregnant, or are breastfeeding after delivery, you should inform your healthcare provider and recommend that you avoid pregnancy within 2 months of vaccination.” It is advised not to vaccinate children under 16 years of age.
The United States and Europe, which have not yet approved the use of the Pfizer vaccine, are paying attention to allergy cases. On the same day, Montseff Slawi, director of vaccines at the White House, said: “The case of adverse events in the UK will be reviewed in the FDA’s expert advisory body on the 10th.” The European Medicines Agency (EMA) also said on the same day that “safety data for vaccines made outside the European Union will be included in the review.”
○ AstraZeneca vaccine approval also slows … Clinical data for older people at low doses is not controversial
Approval of the vaccine by the University of Oxford and AstraZeneca in the UK has also been slowed. The New York Times on the 8th (local time) expressed concern that the US government will not approve the use of the vaccine for this year. The New York Times said: “AstraZeneca is losing confidence in the US FDA. The reason is that there is no explanation for the cessation of two clinical trials in July and September.”
There is also the question of “low dose” with respect to the clinical outcome itself. On the 8th, AstraZeneca published an article in the Lancet medical journal containing the results of the first clinical studies that have been verified (peer-reviewed) by other scientists.
Based on this, the AstraZeneca vaccine was tested to have a 70% preventive effect. The prophylactic effect of clinical participants who received half of the first dose of AstraZeneca vaccine, which was given twice and then the full dose was given one month later, was 90%. The preventive effect was 62%. It is concluded that the prophylactic effect was greater when it was administered at low doses. However, the research team could not reveal why the preventive effect of the vaccine varies depending on the dose and method of administration. The fact that there are no people over 55 years of age in the clinical trial subjects has been flagged as a problem.
The government announced on the 8th that it has insured up to 44 million people with the Corona 19 vaccine. Of these, it has signed a contract with AstraZeneca to supply 20 million doses and 10 million vaccines. AstraZeneca is the largest vaccine supplier along with US pharmaceutical companies Pfizer and Modena.
[ad_2]