[ad_1]
Scheduled to announce the results of negotiations with individual pharmaceutical companies next week
[연합뉴스TV 제공]
(Seoul = Yonhap News) Reporter Shin Jae-woo, Shin-mi Shin = The government has signed a vaccine contract with AstraZeneca, a developer of vaccines for a new coronavirus infection (Corona 19).
A health official said on Day 3: “We recently signed a vaccine supply contract with AstraZeneca, and we plan to announce the status of the entire contract and the amount that will be secured for next week when negotiations with individual vaccine developers conclude. soon”.
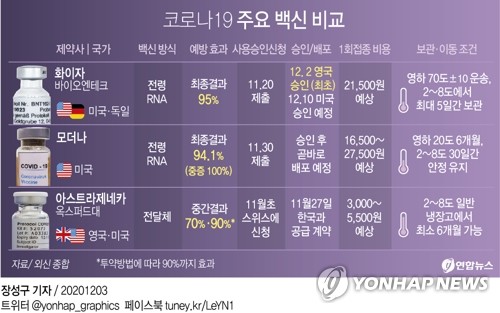
The AstraZeneca vaccine requires two doses and, as a result of the initial data analysis of the phase 3 clinical trial, the prophylactic effect of the vaccine was 70-90% depending on the method of administration.
The company previously promised a supply price of $ 3 to $ 5 (about 3,000 to 5,500 won) per dose (one dose).
The government has negotiated purchase contracts for 5 corona vaccine products19 that have entered phase 3 clinical trials.
AstraZeneca, known to have signed a contract for the first time, is reported to have been negotiating with Pfizer, Johnson & Johnson, Modena and NovaVax, who all approved emergency use in the UK on Day 2.
Pfizer and Moderna have stated that the vaccines they are developing have preventive effects of 95% and 94.1%, respectively. Pfizer’s price per dose is $ 19.5 (about 21,000 won), and Moderna costs between $ 15 and $ 25 (about 16,500-27,000 won). Two doses of the Pfizer and Modena vaccine are also required.
AstraZeneca vaccine is somewhat inferior in its preventive effect, but has the advantage that it can be delivered at 2-8 degrees compared to Pfizer, which must be delivered through a ‘cold chain’ at a cryogenic temperature below -70 ℃. Additionally, you have the advantage that domestic manufacturing is possible by signing a vaccine shipment production contract with SK Bioscience in July.
Initially, the government planned to insure 10 million people through the ‘COVAX facility’, an international project for the joint purchase and distribution of vaccines, and to purchase an additional 20 million people through individual negotiations with global pharmaceutical companies. In addition, more than 3,000 people are expected to eventually be insured.
Health and Welfare Minister Park Neung-hoo recently said: “We are seeking considerably more contracts than 30 million people, or 60% of the total population.” In next year’s budget, 900 billion won has been allocated to purchase vaccines to increase the number of vaccination targets to 44 million.
![[그래픽] Comparison of the main Corona 19 vaccines](https://img0.yna.co.kr/etc/graphic/YH/2020/12/03/GYH2020120300040004400_P4.jpg)
(Seoul = Yonhap News) Reporter Seong-gu Jang = As the new vaccine against coronavirus infection (Corona 19) from Pfizer, an American pharmaceutical company, was approved for emergency use for the first time in the world by the government Britain on day 2, expectations for the end of the pandemic (global pandemic) also rise.
[email protected]
Facebook tuney.kr/LeYN1 Twitter @yonhap_graphics
<저작권자 (c) 연합 뉴스,
Unauthorized reproduction-redistribution prohibited>
2020/12/03 09:04 Sent