
[ad_1]
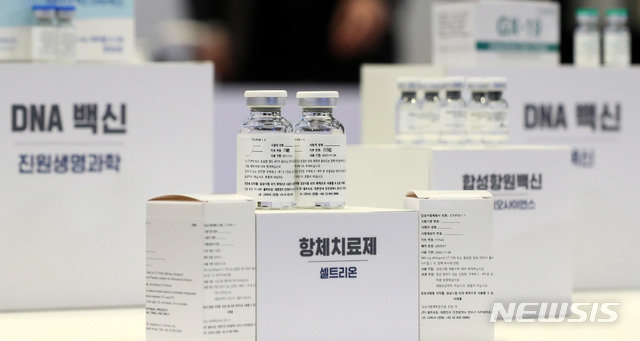
The government predicted that an antibody treatment for a new coronavirus infection (Corona 19), which is developing in Korea, could be approved within this year as soon as possible. However, since clinical trials are still ongoing, it is too early to assess efficacy.
At a regular briefing for Corona 19 held at the Sejong Government Complex on the morning of the 11th, Son Young-rae, head of the Strategic Planning Division at the Accident Recovery Headquarters (Heavy Water), said: “It is Antibody treatments developed by Celltrion are likely to reach a certain level this year. Said.
Human blood is made up of red blood cells, white blood cells, and plasma. Plasma contains antibodies that detect and fight disease-causing pathogens in the body. When antibodies fight a virus, white blood cells rush to eat the virus.
If the blood plasma treatment system is a method to concentrate the plasma of the healed person, then the antibody treatment can be considered to make a treatment by extracting the antibody that fights Corona 19. The Celltrion antibody treatment is currently in the phases 2 and 3 of clinical trials. Celltrion President Seo Jeong-jin said: “As a result of clinical trials, the virus in the body will disappear in 4-5 days.” “Because phase 2 and 3 clinical trials are still in the early stages, we can understand effectiveness through accurate analysis only when data is accumulated,” said Sohn, head of Strategic Planning. “It is still at an early stage to evaluate.”
The government is supporting clinical trials for the development of COVID-19 treatments and vaccines.
“In November, we completed the settlement system and supported a budget of 38 billion won for three projects that completed clinical trials and approvals among the eight targets selected for the first public offering in August.” An additional application target was selected through the second contest. In the future, we plan to provide a new department every two months. ”
In addition, the government is operating a support center to solve the difficulties of companies developing the COVID-19 treatment and vaccine.
In-depth consultancy is being carried out with the corresponding ministries, and since last May, 56 companies have been carried out a total of 16 times.
“Today, we are working hard to respond quickly to receiving 244 major requests from companies, such as the use of candidate efficacy testing facilities, rapid screening of clinical trials and pre-consultation, and 223 cases are handled.” (11th) also plans to conduct the 17th in-depth consultation for three companies ”.
[서울=뉴시스]Copyright by dongA.com All rights reserved.