[ad_1]
The Central Pharmacy Review Committee delivered the final decision to the Agency for Disease Control in Europe
Decision made through the Final Inspection Committee of the Ministry of Food and Drug Safety and the Vaccination Committee of the Korea Disease Administration
“The whole plan breaks down when vaccination is reserved for the elderly”, “Anyway I have to start vaccinating”

New Infectious Disease by Coronavirus (Corona 19) since the Ministry of Food and Drug Safety and the Central Pharmaceutical Review Committee (Corona 19) withheld the judgment on whether to inoculate the AstraZeneca (AZ) vaccine for people over 65 years of age or more and handed the decision to the Korea Centers for Disease Control and Prevention. I am concerned about the interruption of the vaccination plan.
The Ministry of Food and Drug Safety (KFDA) approved vaccination against AstraZeneca for people over 18 years of age as a result of the Central Pharmacopoeia meeting, the second stage of expert advice for the AstraZeneca vaccine on day 5).
The Ministry of Food and Drug Safety is undergoing a “triple” expert advice procedure leading to the COVID-19 Vaccine Safety and Efficacy Verification Advisory Group (Verification Advisory Group), the Central Pharmacopoeia and the committee final inspection.
◇ Central Pharmacopoeia “There are insufficient data on the effect of vaccination in the elderly, so it must be decided carefully”
The Central Pharmacopoeia said: “There is not enough data on the effectiveness of vaccines against AstraZeneca over 65 years, so it must be decided carefully,” and did not reach a conclusion on the vaccination of the elderly, a key issue.
This is a step back from the fact that the verification advisory group, which is the first expert advisory meeting on Day 1, issued a majority opinion that all age groups over 18 should be vaccinated.
The analysis suggests that this position is linked to the situation in which the controversy over the vaccination of older people in Europe is intensifying.
On the 29th of last month, the European Union (EU) officially approved the conditional sale of the AstraZeneca vaccine, but Germany and France recommended vaccination only for those under 65 years of age because there is insufficient data to demonstrate the efficacy of the vaccine for those under 65. elderly.
Furthermore, Belgium has lowered the age of those vaccinated to less than 55 years.
Additionally, Italy initially recommended preferential use for those under 55, but recently revised the view that even those over 55 can receive the vaccine if they are healthy.
Although not a member of the EU, the Swiss government has suspended approval of the AstraZeneca vaccine and required additional data to be submitted.
◇ Possible interruption in the vaccination plan … “If you cannot vaccinate the elderly, your vaccination plan will be ruined”

Immediately after the decision of the Ministry of Food and Drug Safety and the Central Pharmaceutical Affairs Deliberation, the Agency for Disease Control and Prevention said: “After final approval and examination by the Ministry of Food Safety and Medications, the results were reflected and after the review of the ‘Advisory Group of Experts in Vaccines of the Crown 19’ and the ‘Committee of Experts in Vaccination’, the vaccination plan of AstraZeneca will be finalized ”.
However, amid the controversy in Europe, even the Chinese medicine center raises the need for a careful decision, so it is hoped that the Agency for Disease Control and Prevention cannot easily draw conclusions.
Also, if the schedule of the final inspection committee has not been specified, which is the final stage of deliberation of the Ministry of Food and Drug Safety, and the decision on the vaccination committee of the Agency for Disease Services of Korea, the time remaining until the start of vaccination at the end of this month is very short.
Vaccination can be harmful from the start.
Since the end of this month, the government has launched a vaccination plan against medical personnel such as doctors, nurses, hospital workers, etc. serving Corona 19 patients, and residents of nursing hospitals and facilities with large numbers of the elderly. people over 65 years old.
Among them, it is known that corona 19 medical staff are almost caught receiving the Pfizer vaccine secured through the ‘COVAX facility’, an international project for joint purchasing and distribution of vaccines, and the elderly are receiving the AstraZeneca vaccine.
If it is decided that the AstraZeneca vaccine cannot be vaccinated for the elderly, the initial vaccination plan will have to be incorrect.
In this case, only medical personnel will receive the vaccine first, and for the elderly, the vaccination may need to be delayed until another vaccine such as the one in Modena comes in.
Currently, the timing of introduction of vaccines supplied directly by each pharmaceutical company in Korea is Q1 for AstraZeneca (for 10 million people), Janssen (for 6 million people) and Modena (for 20 million people) of the second quarter and Pfizer (for 10 million people). million people) .They are held from 3Q.
Even if the decision is made to inoculate the elderly with the AstraZeneca vaccine, controversy about its efficacy is likely to continue and there will also be interruptions in the vaccination process.
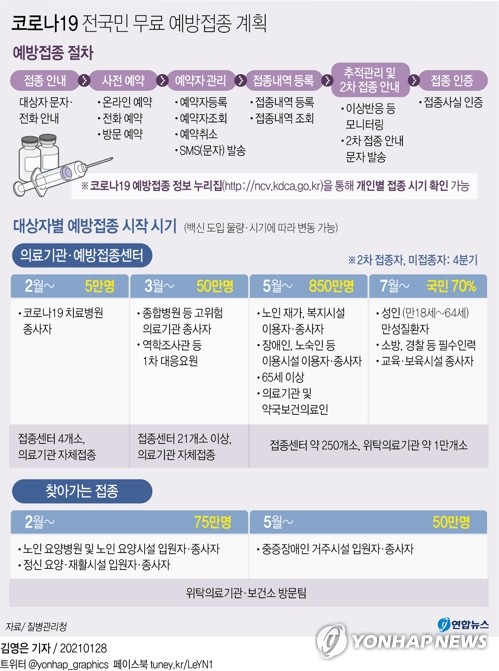
In the end, the first vaccination was completed for all citizens in September in the order of seniors in medical staff and nursing hospitals in the first trimester, seniors 65 years and older in the second trimester, and adults 18 to 64 years in the third. It is also impossible to rule out the possibility that the entire vaccination plan to form ‘not cruise capable.
◇ “The role of the Ministry of Food and Drug Safety in determining the vaccination permit should be my role”, “Anyway, I have to start vaccinating”
An expert who requested anonymity said: “The original AstraZeneca vaccine had to be given first to the elderly, nursing homes and nursing homes before other vaccines. If the vaccine cannot be given to people over 65, the plan vaccination will be ruined. ” “The Vaccination Committee is not an organization that decides whether or not to approve drugs, and it is a matter for the Ministry of Food and Drug Safety to decide,” he said.
Professor Kim Woo-joo, Professor of Infectious Medicine at Kodae Guro Hospital, said: “The decision of the Ministry of Food and Drug Safety is ambiguous. The Ministry of Food and Drug Safety shares the role of establishing the scope of use. of the vaccine, and KFDA should play a role. ” Said.
Professor Kim said: “Although there is a final review committee of the Ministry of Food and Drug Safety, it is a situation that can be celebrated, and the decision on vaccination from the age of 65 will depend on the discussion of the vaccination committee. “” The number of elderly clinical participants is 660, which is too small compared to Pfizer and Modena, so we need to assess safety. “
Based on data from the UK, where vaccination for the elderly has already started, many opinions have suggested that there will be no major disruption to the national vaccination schedule.
Jeong Jae-hoon, professor of preventive medicine at Gachon University School of Medicine, said: “It seems that the Ministry of Food and Drug Safety is awaiting evidence (data) from a very cautious point of view. In the Kingdom United, vaccines are already being applied. Made for the elderly. “I think the data will accumulate.”
Professor Jeong said: “It is important that the UK and European authorities have approved the AstraZeneca vaccine,” he said. “There is an alternative to the Pfizer vaccine in countries that are cautious about vaccination with AstraZeneca, but since Korea does not have an alternative, we calculate the risks and benefits. When it does, it should start the vaccination somehow.”
Professor Kimoran from the National Cancer Center also said: “We have already started vaccinating AstraZeneca on a large scale in the UK, and there are many people who have been affected,” he said. “If there was an adverse reaction, it would have been reported, but there was no such report.”
Professor Ki explained: “The issue of safety should be considered as an adverse reaction. In the clinical trial of (AstraZeneca Vaccine), there was a concern that there were few subjects in the elderly, but it appears that safety has been confirmed as There are currently many vaccines registered and no adverse reactions have been reported.
Professor Ki responded that some European member states have restricted the vaccination of the AstraZeneca vaccine to the elderly, “each country has implemented different policies depending on which vaccine is available and in what quantity.”
Regarding some views that the safer NovaVax vaccine should be given to the elderly, he said: “The first target of vaccination is the elderly, but you should not wait for NovaVax to arrive.” It aims to reduce it, so it is best to get the vaccine available for the elderly who are at high risk of death. “
/ yunhap news