[ad_1]
Corona 19 vaccines in national industry development
Most Traditionally Manufactured Protein Vaccines
The Pfizer, Moder or RNA vaccines surprised with positive results
“There will be demand for highly universal vaccine products”
Follow-up strategy of the main manufacturers
“Production on consignment of genetic vaccine, national development of protein vaccine”
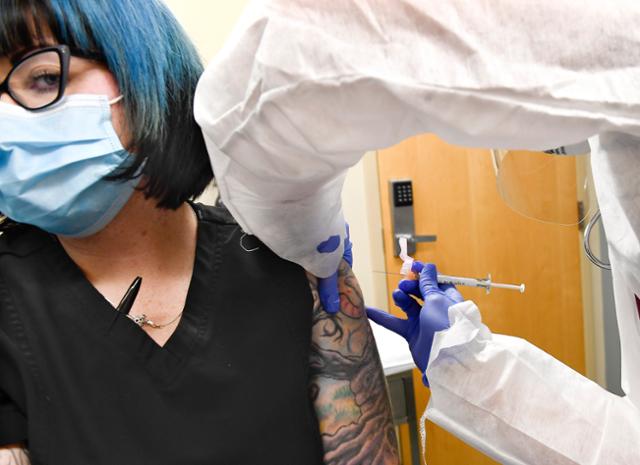
On July 27, in Harpersville, New York, a participant in a clinical trial of a COVID-19 vaccine will receive a candidate vaccine from US biotech company Modena. Harper Brownsville = AP / WIRE
The multinational pharmaceutical company Pfizer and the American biological company Modena announced positive interim results of clinical trials for vaccines against novel coronavirus infection (Corona 19) and announced an urgent request for approval. Most companies predicted that there would be limitations in the commercialization of a new technology, a genetic vaccine, and they turned to traditional protein vaccines because the clinical effect of genetic vaccines exceeded expectations. Experts emphasize, however, that the genetic vaccine may be available from the first national companies and, finally, the stamps required in traditional manufacturing methods to make universal vaccine supplies necessary to accelerate development.
According to the pharmaceutical industry and scientific community on the 18th, the COVID-19 vaccine candidates from Korean pharmaceutical companies are currently in the early stages of human clinical trials or about to enter when the speed of development is early. If you are confident that year 1 and 2 clinical trials in healthy people determine safety, SK Bioscience submitted a phase 1 permit last month, KFDA. Jinwon Life Science also applied for phase 1 and 2 clinical trials to the Ministry of Food and Drug Safety earlier this month, and UBiologics is preparing to apply.
In addition to Eno HK yen (ex CJ Healthcare), the new fusion virus is brought to the vaccine candidates from the Korea Chemical Research Research Institute and the preclinical study (laboratory animals) continues. GC Green Cross is discovering vaccine candidates, and LG Chem is also looking for candidates in cooperation with biological companies Celed and Smagen.
Compared to foreign companies such as Pfizer and Modena, which have already announced the intermediate results of phase 3 clinical trials, and AstraZeneca, Johnson & Johnson and NovaVax, which are also in phase 3 clinical trials, the speed of development of the national Corona 19 vaccine is significantly inferior. With extensive clinical experience and workmanship, funding from a global pharmaceutical company that makes the reality of the situation non-vaccine-friendly received the full support of national health authorities and easy-to-follow national companies.
The different principles of the shots are also a factor in the speed difference. The more advanced Pfizer and Moderna vaccines are primarily genes (RNA). It is made by chemically synthesizing the RNA of the Corona 19 virus, and it is a technology that domestic companies can do enough because it is relatively easy to mass-produce. However, it is predicted that if only the initial 19 Corona RNA virus were the predominant effect, it would remain at 40% to 50%. Consequently, the national pharmaceutical industry has chosen to create a stable vaccine in the way it has been doing rather than compete with global companies to develop a new RNA vaccine that has never been commercialized. Corona 19 vaccines from most companies, except God jenek epicenter Life Sciences, are composed primarily of corona 19 virus proteins (antigens) created by recombinant DNA technology.
However, as the RNA vaccines from Modena and Pfizer produced an effect that exceeded expectations in intermediate results, domestic companies were forced to consider whether to continue developing the vaccine with a lot of time and money. Now, it is not easy to suddenly change the direction of development towards RNA vaccines. An official at a vaccine manufacturer noted that “although RNA vaccines can be developed and produced, it is difficult to participate in and manage the trillion-dollar clinical trials.”
Even if the RNA virus is released The academic world is hearing the voice that domestic manufacturers should continue to develop protein vaccines. Eventually, the expected demand would lead to a vaccine using traditional manufacturing processes constituting a universal vaccine supply because RNA is relatively complicated and expensive for temperature conditions. The editor-in-chief of the Honggijong Vaccine Society (Konkuk University) has predicted that “fasting for the supply stream is the required RNA vaccine, but two, it will be the recombinant virus that will arrive in three years.” Domestic vaccine manufacturers are autoprotein vaccines, while contract manufacturing of foreign genetic vaccines may establish a strategy to go after development.
The crown 19 jenek God complete round life sciences vaccine is a genetic vaccine that uses DNA instead of RNA. When injecting DNA vaccines, electrical stimulation or special syringes are required, so acceptance of inoculation is low.
I am of short stature reporter [email protected]
Subscribe to the Hankook Ilbo News Naver channel

Balance to see the world, the Hankook Ilbo Copyright © Hankookilbo
[ad_2]