[ad_1]
The government disclosed that there were no quality abnormalities as a result of testing the influenza (flu) vaccine exposed to room temperature during distribution. However, it was confirmed that some of the vaccines were transported below zero degrees, which further revealed problems in the distribution process.
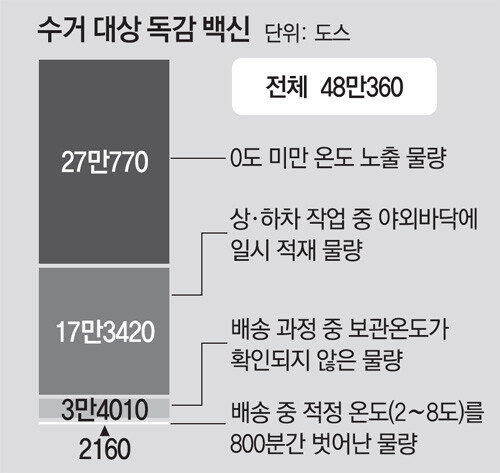
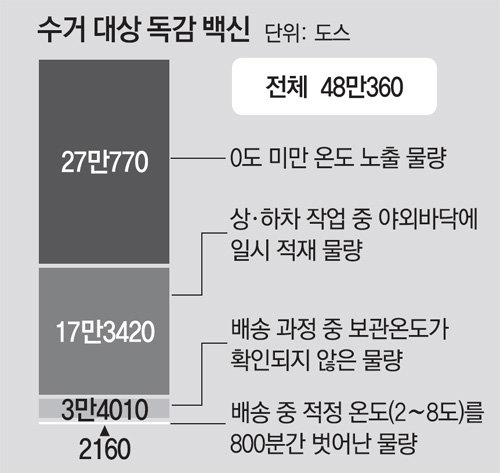
On the 6th, the Korean Centers for Disease Control and Prevention (DDS) and the Ministry of Food and Drug Safety (MFDS) announced the results of a survey and quality assessment of the vaccine distribution process. influenza. Of the 5.37 million doses transported by Shinsung Pharm and its partner DEL Pharm from September 10-21, the vaccine exposed to room temperature during the loading and unloading process was confirmed to be about 170,000 doses. In addition, 2000 degrees exceeded the proper storage temperature (2 to 8 degrees) for 800 minutes (13 hours 20 minutes) during transportation. The amount of water for which the storage temperature was not confirmed at all was 30,000 doses. Some vaccines were exposed to temperatures below zero degrees. As such, a total of 480,360 degrees Celsius was exceeded or the proper temperature was not maintained. The government collected 2,100 doses of vaccines delivered to 14 regions as samples and performed sterilization tests for 14 days in conditions outside the proper temperature, finding that they were all fine.
Furthermore, there were no anomalies in the results of a separate investigation of 12,736 doses of vaccines produced by eight companies that signed a procurement contract with Shinsung Pharmaceutical. All eight products were exposed to 25 ° C for 24 hours and there were no quality anomalies. The Agency for Disease Control and Prevention said: “There were no cases of exposure to the 37 degree environment among the vaccines that were a problem during distribution this time.”
However, through this research, it was confirmed that 27,770 doses of vaccine were shipped at sub-zero temperatures. A new problem has been discovered in the distribution process. Chung Eun-kyung, director of disease management, said: “When frozen, there are cases of cloudy deposits in the vaccine. In that case, the syringe may be clogged or a real problem may occur at the inoculation site. “However, it does not mean that it was frozen in quantity and that there were no safety concerns even for products stored below 0 degrees. The government decided to collect the quantities that were found with problems in the transportation process. It was confirmed that there was no anomaly as a result of the quality test, but the possibility of an “aqueous vaccine” that the vaccine was ineffective did not could be excluded entirely. Items subject to collection were delivered in 11 areas, including Seoul, Daegu, Incheon, Gwangju, Gyeonggi, Gangwon, Chungnam, Jeonnam, Gyeongbuk, Gyeongnam, and Jeju. Of these, 554 vaccines were found in 7 regions. The government plans to propose measures for these people through the Vaccine Review Committee. Revaccination and reimbursements are also expected to be considered. The government plans to resume vaccination starting on the 12th. Free vaccinations will resume for high school students (16-18 years old) and high school students (13-15 years old), whose vaccination was suspended after the 22nd of last month. Initially, the intensive vaccination period was September 22-29 for high school students and October 5-12 for high school students. Primary school students (7 to 12 years old) and pregnant women resumed vaccination from 25 last month.
Some citizens still couldn’t hide their anxiety. Shinmo (37, female) who lives in Yangcheon-gu, Seoul, said: “I am concerned that some vaccines are actually exposed to room temperature. Jeong Ki-seok, professor of infectious medicine at the Sacred Heart Hospital of the University of Hallym (former director of the Centers for Disease Control) said: “Of the 5.37 million doses administered by Shinsung Pharmaceutical, the sample analyzed by the Ministry of Food and Drug Safety is only 14,000 doses, so it’s not 100% safe. He noted that since multiple organizations are in charge of vaccine management, blind spots will inevitably occur. “
Image [email protected] Go to reporter’s page>Reporter Dongwoong Kang
Copyright by dongA.com All rights reserved.