[ad_1]
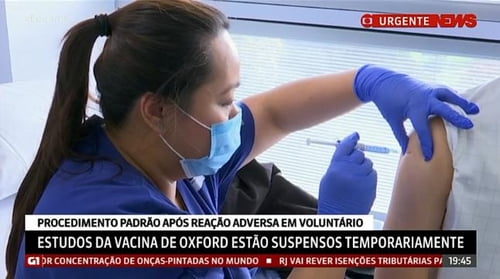
A global clinical trial of a new vaccine against coronavirus infection (Corona 19) being developed by the British pharmaceutical company AstraZeneca was temporarily suspended due to potential side effects, and no adverse side effects were reported in a phase 3 clinical trial in Brazil. said the Federal University of Sao Paulo.
According to Brazilian media, on the 9th (local time), a phase 3 clinical trial with the AstraZeneca vaccine has been in progress in Brazil with 5,000 volunteers so far, and there have been no reports of serious side effects.
The Federal University of Sao Paulo, which participated in the clinical trial, explained in a statement that “many of the applicants received a second dose of the AstraZeneca vaccine and the results are as expected.”
At the same time, he said that a global clinical trial for a candidate vaccine will be temporarily suspended as a possible side effect of the disease outbreak of the AstraZeneca vaccine clinical trial participants is “common in clinical studies related to drug development. “.

However, the Brazilian Ministry of Health is concerned about the possibility of interruption of the Corona 19 vaccination plan.
The Health Ministry signed a contract to purchase 100 million doses of vaccine with AstraZeneca in late July and set a special budget of 2 billion reais (about 434 billion won) for it.
The Ministry of Health is also pushing for a plan for the Osbaudu Cruz Foundation (Fiocruz), a biological research and development organization, to produce its own corona 19 vaccine starting in April next year through the transfer of technology from AstraZeneca.
Acting Health Minister Eduardo Pazuelu attended the Senate-Home Joint Commission in the middle of last month and said that the vaccine that AstraZeneca is developing with the University of Oxford is the best option.
The Pajuelu agency said the day before that the Corona 19 vaccine will be insured in January of next year, and that the vaccine will be administered to all citizens.
The Butantang Research Center, under the government of São Paulo, has been conducting phase 3 clinical trials of the ‘Coronavac’ vaccine being developed by China Sinovac Bio Co., Ltd. since July 21.
Sao Paulo Governor Juan Doria announced that he will begin vaccinating Sao Paulo residents within this year once the safety of the ‘crown bag’ is proven.
On the other hand, the situation of Corona 19 in Brazil has been showing a clear calm in recent years.
Based on data from the Ministry of Health, the accumulated number of confirmed cases up to the previous day was 4.62.73 and the accumulated death was 127,000.
The new confirmed cases remained in the 10,000 range for 3 consecutive days from day 6 to the previous day, and the daily number of deaths was less than 1,000 for 6 consecutive days from day 3 to the previous day.
Brazil has the third-highest cumulative number of confirmed cases after the United States and India, and the second-largest cumulative death after the United States.
/ yunhap news