
[ad_1]
“India and the UK are likely to approve the use of the AstraZeneca vaccine”
Expert meeting scheduled shortly after UK approval … Vaccination promotion starting next month
18,000 new confirmed cases … Minimum in 5 months from the beginning of July

(New Delhi = Yonhap News) Correspondent Kim Young-hyun = The Indian government plans to approve the emergency use of a new vaccine against coronavirus infection (Corona 19) jointly developed by AstraZeneca and the University of Oxford after the UK.
An Indian health authorities official said: “As soon as the UK Medicines Regulatory Authority approves the AstraZeneca vaccine, we will hold a meeting of the Central Pharmaceutical Standards Administration (CDSCO) Committee of Experts and thoroughly review the Safety and immunogenicity data of this vaccine Indian media like PTI News reported on day 27.
The official added that the AstraZeneca vaccine is expected to be approved for emergency use in India and distributed accordingly.
To date, no country has approved the emergency use of the AstraZeneca vaccine. The British newspaper Telegraph reported on the 26th that the first approval could be withdrawn in the UK from the 27th.
Currently, six vaccines are in clinical trials in India and three other vaccines are known to be in clinical trials.
Among them, three companies, including the Serum Institute (SII), which is conducting clinical trials of the AstraZeneca vaccine, the local pharmaceutical company Barat Biotech and the US pharmaceutical company Pfizer, have requested the urgent use of the vaccine from the Indian authorities.
PTI said it will take a little longer to approve the emergency use of the vaccine from Barat Biotech and Pfizer. In particular, it is reported that Pfizer has not made a submission on approval of the use to the Indian authorities.
India plans to start vaccinating about 300 million people starting next month, when approval for the emergency use of the vaccine is withdrawn.
Ten million people in the medical and health sector are the top priority targets for vaccination, and vaccination will be expanded to 20 million people, including police, military personnel, and local government employees, as well as patients with complications of 50 years or more and under 50 years.
The SII has already finished producing 50 million doses in preparation for urgent approval. The SII plans to produce 400 million doses of the Corona 19 vaccine by next July.

Furthermore, India is tracking all those who have arrived from the UK in the last month to prevent the spread of the highly contagious Corona 19 variant in their country.
At present, in New Delhi, Telangana, Maharashtra, etc., dozens of people have recently been confirmed infected after entering the UK.
Two of these people, in particular, escaped from the New Delhi quarantine facilities, went to their home provinces and were arrested by the authorities.
Previously, the British government implemented an emergency containment measure on the 19th when the corona19 variant of the virus spread rapidly in the southeast of England, including the capital London.
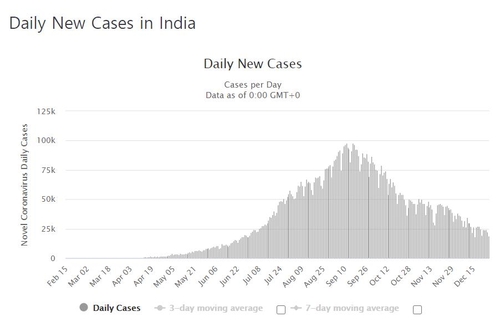
Meanwhile, according to the Indian Ministry of Health and Family Welfare, the cumulative number of confirmed corona 19 cases in India that day was 18,7,850, an increase of 18,732 from the previous day.
The global ranking for the cumulative number of confirmed cases is second only to the United States (1.943 million, based on world ohms), but the spread has slowed significantly recently.
In September, the number of new confirmed cases per day reached 100,000. The number of confirmed cases per day on this day is the lowest in about 5 months since the beginning of July.
[email protected]
(The end)
<저작권자(c) 연합뉴스, 무단 전재-재배포 금지>
Copyrightⓒ Korea Economic Daily TV. All rights reserved. Unauthorized reproduction and redistribution prohibited
[ad_2]