[ad_1]
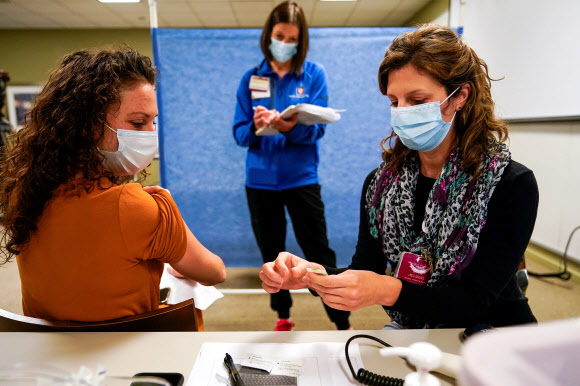
An employee of a nursing home in Indianapolis, Indiana, USA, participates in Pfizer’s pre-exercise for COVID-19 vaccination on the 11th (local time). 2020.12.12
Reuters Yonhap News “style =” padding: 0px; margin: 0px “>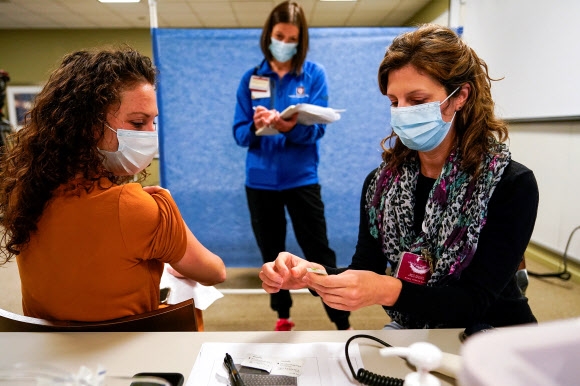
▲ Pfizer vaccination in the US
An employee of a nursing home in Indianapolis, Indiana, USA, participates in Pfizer’s pre-exercise for COVID-19 vaccination on the 11th (local time). 2020.12.12
Reuters Yonhap News
On the 12th (local time), the Vaccination Advisory Committee (ACIP), an advisory body to the Centers for Disease Control and Prevention (CDC), recommended the use of Pfizer’s COVID-19 vaccine.
Bloomberg News and CNN reported that the advisory committee held a meeting and voted to recommend that Americans over the age of 16 inoculate the COVID-19 vaccine jointly developed by Pfizer and German Bioentech.
The vote was “11 to 0” and it was considered appropriate to use this vaccine in the general public. Three of the advisers abstained from voting due to a conflict of interest.
The advisory committee also recommended that a doctor / nurse monitor the condition for 30 minutes after vaccination for people who have had hypersensitivity or allergic reactions.
For pregnant women, breastfeeding women, and people with compromised immune systems, they advised letting them decide whether to get the vaccine.
The recommendation of the advisory committee for vaccines is a procedure that should be followed before inoculating the newly developed vaccine.
If CDC Director Robert Redfield accepts this recommendation and officially approves the use of the vaccine, then people can get the vaccine.
CNN reported that Redfield is expected to approve the ACIP recommendations in a few hours.
Prior to the advisory committee’s recommendation, the Food and Drug Administration (FDA) issued an emergency use approval (USA) for Pfizer’s Corona 19 vaccine on the 11th day of the previous day. CDC can decide whether to recommend use of the vaccine after the FDA has approved it for emergency use.
If the CDC finally approves Pfizer’s COVID-19 vaccine on this day, the vaccine is expected to start on day 14 in the United States.
On day 2 (local time), a freighter heading from Brussels Airport in Belgium to O’Hare International Airport in Chicago, Illinois, USA, carries the Corona 19 vaccine developed by Pfizer. 2020.12.13
Reuters Yonhap News “style =” padding: 0px; margin: 0px “>
▲ Pfizer vaccine destined for the US.
On day 2 (local time), a freighter heading from Brussels Airport in Belgium to O’Hare International Airport in Chicago, Illinois, USA, carries the Corona 19 vaccine developed by Pfizer. 2020.12.13
Reuters Yonhap News
General of the Army Gustav Ferna, Chief Operating Officer (COO) of the U.S. Administration’s Corona 19 Vaccine Development Program, Ultra High Velocity Operations, announced that Pfizer’s Corona 19 vaccine will begin to reach 145 delivery locations in the US from the morning of Monday 14.
CDC previously recommended that health officials make vaccination a priority for health care workers and residents and staff of long-term care facilities.
Consequently, Reuters predicted that hospitals are expected to urgently begin vaccinating employees from the 14th already in the region.
Reporter Shin Jin-ho [email protected]
