
[ad_1]
It was found that with the impending COVID-19 vaccination in major countries, at least 12 countries have confirmed purchases of more than three types of vaccines so far. The United Kingdom and Canada have signed a contract for the purchase of 7 vaccines, the United States, EU (European Union) and Indonesia 6, Australia and Mexico 4, and Japan, India, Brazil, Chile and Ecuador 3 types of vaccines.
Korea signed a purchase contract for a single AstraZeneca vaccine
7 species were insured from the UK and Canada, 4 from Australia and 3 from Japan.
Expert, “To prepare for an unexpected situation, you must buy several”
The JoongAng Ilbo calculated the Corona 19 vaccine types that have completed a purchase contract for each country based on the count of the country’s Corona 19 vaccine purchase status (as of the last 4 days) at the Innovation Center in Global Health from Duke University.
Experts “Although vaccination is careful, we should have ensured several vaccinations before”
At present, 12 countries are likely to adopt a first-to-market strategy to ensure multiple vaccines for each type, as it is unclear which vaccine will be safe and effective.
![So far, at least 12 countries appear to have purchased more than three types of vaccines. [로이터=연합뉴스]](https://pds.joins.com/news/component/htmlphoto_mmdata/202012/07/6a664cb5-763f-47b8-b4e0-8c41a12ddd69.jpg)
So far, at least 12 countries appear to have purchased more than three types of vaccines. [로이터=연합뉴스]
Australian Prime Minister Scott Morrison said more than 134 million doses of the four vaccines have been secured. “Our strategy is to put our people in front of the (vaccine) queue.” “We put all the eggs in one basket.” We are not doing this (which means we are reducing risk through distributed purchases) and we will introduce additional vaccines if the experts recommend it. “
Canadian Prime Minister Justin Trudeau commented on the strategy to secure vaccines from various developers: “It is to convince the public that there will be tens of millions of vaccines available, regardless of which vaccine is most effective and which comes first.” Explained.
The government, which has started buying the corona vaccine, is also speeding up negotiations. However, the vaccine, which the government says has currently signed a purchase contract, is a type from AstraZeneca of the UK, which is scheduled to be produced on commission in Korea. Recently, with 500 to 600 confirmed cases per day, there is concern that Korea is behind in the vaccination battle.
![UK medical staff are simulating the last 4 days before Pfizer's vaccination next week. [AP=연합뉴스]](https://pds.joins.com/news/component/htmlphoto_mmdata/202012/07/8b06091c-75c0-474f-8527-07fcca2a839f.jpg)
UK medical staff are simulating the last 4 days before Pfizer’s vaccination next week. [AP=연합뉴스]
Experts advise that even if you start vaccinating against Corona 19 carefully, you should have several vaccinations in reserve for unexpected problems.
Kim Woo-joo, professor of infectious medicine at Korea University Guro Hospital, said: “If there is a problem with the vaccine, you need to buy vaccines with different manufacturing methods, such as mRNA (Pfizer Modena), viral vector (AstraZeneca ) and protein recombination (NovaVax). I can. “
Professor Park Eun-cheol from Yonsei University’s Department of Preventive Medicine also noted that “if we rely on just one or two vaccines, we need to distribute and buy the number of vaccines,” he said.
Australia, which has fewer confirmed cases than Korea, also buys 4 types
In fact, in the case of the UK, although there is a domestically developed AstraZeneca vaccine, a total of 7 types have been secured, including the US-developed Pfizer / Moder vaccine. The UK is the first in the world to receive the Pfizer vaccine approved for emergency use, beginning on the 7th (local time).
![The Pfizer vaccine, which begins vaccination on day 7 in the UK, is stored in a freezer somewhere.[로이터=연합뉴스]](https://pds.joins.com/news/component/htmlphoto_mmdata/202012/07/6c019292-eae8-43c3-8bdb-870409b48347.jpg)
The Pfizer vaccine, which begins vaccination on day 7 in the UK, is stored in a freezer somewhere.[로이터=연합뉴스]
In Canada, in addition to the AstraZeneca vaccine, Pfizer, Modena, which has led to phase 3 clinical trials, Johnson & Johnson (USA), Sanofi (France), Medicago (Canada), NovaVax (USA). ) Ongoing or before phase 3 I purchased the Corona 19 vaccine. So far, you have purchased 9.5 servings per person.
In an interview with Global News, Professor Maxwell Smith of Western University in Canada told Global News that “overorder” is a precautionary measure when it is not clear which vaccine will be successful and if all orders will be available.
Australia has 27,963 confirmed cumulative cases of corona19 (as of the 6th world meter), which is 9,500 fewer than Korea. There are about 10 confirmed cases per day from last month to the last 5 days. Still, AstraZeneca, Pfizer, NovaVax and the University of Queensland (Australia) have signed a contract to purchase a COVID-19 vaccine.
Japan, which has decided to give the corona 19 vaccine free to all citizens, has confirmed the purchase of the AstraZeneca, Pfizer and Modena vaccines. New Zealand, Bangladesh, Israel, Egypt, and Argentina have two vaccines.
The Pfizer vaccine, which Korea did not buy, has purchase contracts in 18 countries
In Korea, considering the time it takes for the Ministry of Food and Drug Safety to approve the use after the final AstraZeneca Phase 3 result and the timing of the supply of supplies, it is noted that vaccination will be possible only in the third quarter of the next year. In addition, AstraZeneca should continue with additional clinical trials, as it emerged that the researchers made a mistake after the announcement of the interim Phase 3 results last month, and a controversy arises over reliability.
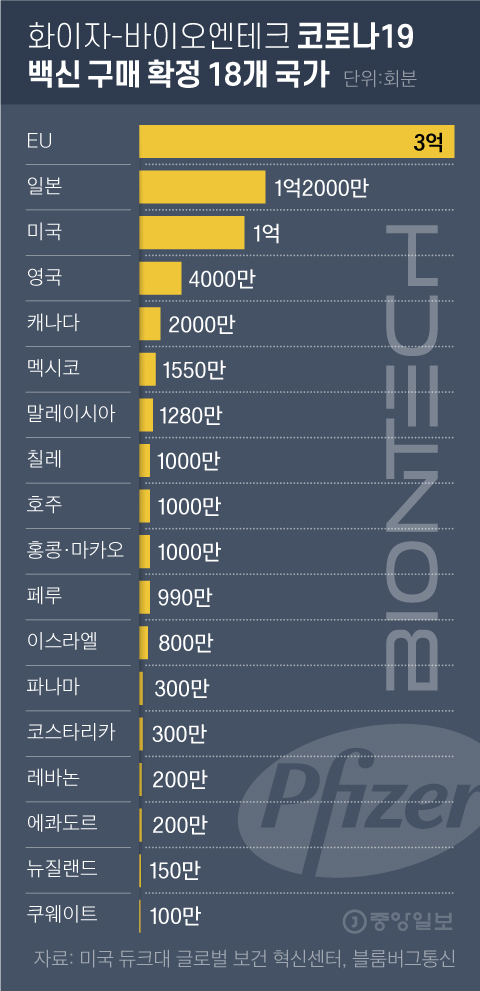
18 countries confirmed to purchase the Pfizer-Bioentech COVID-19 vaccine. Graphic = Reporter Cha Junhong [email protected]
Professor Kim Woo-joo noted: “With the high possibility of supply in purchase order, we still lack the type and quantity of vaccines that we have bought, and the timing of supply may be delayed compared to other countries.” Following “Also, from January to February next year, we should have gotten a vaccine in advance because we can know which vaccine is safe and effective based on the results of vaccines in the US and the UK,” he said.
18 countries (including regions), including Malaysia, Israel, New Zealand and Lebanon, have confirmed the purchase of Pfizer vaccines, with which the government has not yet signed a purchase contract.
![Purchase of Pfizer vaccine was contracted in 18 countries (including regions). [AFP=연합뉴스]](https://pds.joins.com/news/component/htmlphoto_mmdata/202012/07/06b7a31f-3670-4c36-ac22-3c2bee244f59.jpg)
Purchase of Pfizer vaccine was contracted in 18 countries (including regions). [AFP=연합뉴스]
On the 3rd, Bloomberg News reported that Hong Kong and Macau also purchased 10 million doses of the Pfizer vaccine. The Pfizer vaccine is expected to be approved for emergency use in the United States and Canada. Bahrain has approved the use of the Pfizer vaccine for the second time after the UK. However, he did not disclose the amount of the purchase.
The biggest problem is that even countries in a rush to buy vaccines are expected to have a hard time sourcing vaccine quantities this year. Supply capacity does not adapt to demand.
On the 4th, CNN announced that the US Centers for Disease Control and Prevention (CDC) selected 24 million medical personnel and nursing home patients as primary vaccination targets. It was reported to be limited to (20 million people).
Reporter Lim Sun-young [email protected]
[ad_2]