[ad_1]
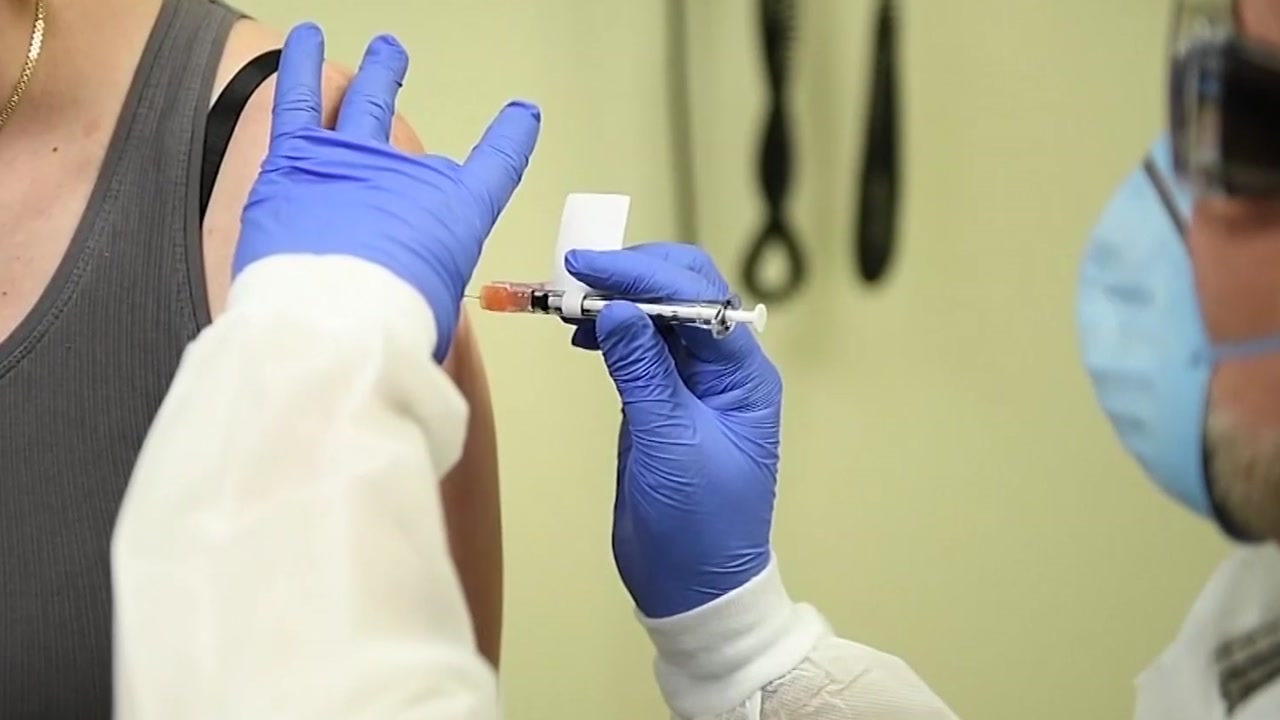
EU Commissioner calls on Member States to prepare for vaccination
European Medicines Agency “The clinical trial of the Corona 19 vaccine is very positive”
European Medicines Agency, Pfizer, Moder, vaccine, etc. in progress
US Vaccine Official “Available to 110 million people in February next year”
[앵커]
The European Union, said the EU commissioner, could start vaccination within a year. In the United States, 100 million people will be vaccinated by February next year, the director of vaccine development said. Reporter Won-bae Kim reports. The head of the European Union and the EU administration, Ursula Fonderaien, attended the European Parliament on the 25th local time and said she can likely start vaccinating Corona 19 before the end of next month. Pon der Raien’s chief executive urged EU member states to prepare for vaccination. In this regard, the Commissioner of the European Medicines Agency, Emma Cook, said in an interview with Irish RTE Radio on the 25th local time that she hopes to make a positive scientific evaluation of the COVID-19 vaccine before Christmas. “What we’ve been hearing right now are the results of clinical trials, and these are very positive,” Cook said. The European Medicines Agency is conducting a joint review of the COVID-19 candidate vaccine jointly developed by the US pharmaceutical company Pfizer and the German Bioentech, as well as the US pharmaceutical company Modena and the UK’s Oxford University and the company. multinational pharmaceutical company AstraZeneca. . The Companion Review is a procedure for rapidly evaluating drugs or vaccines from promising clinical trials in public health emergencies such as the global COVID-19 epidemic. Monsef Slawi, executive director of the ‘Operation Super Fast’ team that oversees the development of the Corona 19 vaccine in the United States, appeared on CBS on the 25th local time, and said that up to 100 million Americans will be vaccinated next February. year. This is YTN Kim Won-bae.