[ad_1]
![[생명공학정책연구센터 제공]](https://pds.joins.com/news/component/htmlphoto_mmdata/202011/19/3d55c2dd-7ca4-4279-b2a3-4e9cc8176dec.jpg)
[생명공학정책연구센터 제공]
Amid the high expectations of the spread of vaccines, attention is being drawn to the prices of the main vaccines, as the results of the new vaccine against coronavirus infection (Corona 19) that it is developing have been shown the American pharmaceutical company Pfizer and Modena have 95% preventive effects.
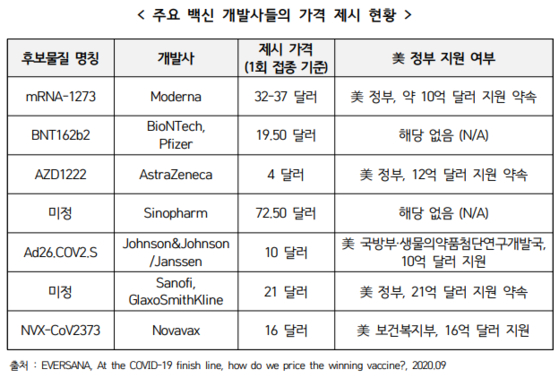
According to the ‘Corona 19 Vaccine Price Outlook and Pricing Model’ published by the Center for Biotechnology Policy Research, the prices suggested by leading vaccine developers are as low as $ 4 (about 4,500 won) per dose and as high as $ 72.50 (89,000 won). Won).
The most affordable vaccine is ‘AZD1222’, a corona 19 candidate vaccine developed by AstraZeneca, UK. AstraZeneca offered the vaccine at $ 4 per dose.
The ‘Ad26.COV2.S’ vaccine from the US pharmaceutical company Johnson & Johnson (J&J) ranked the next cheapest vaccine after AstraZeneca at $ 10 (11,100 won). The two pharmaceutical companies have announced that they will not aim to generate revenue through vaccines during the Corona 19 pandemic (a global pandemic).
On the 16th (local time), Moderna, which is raising expectations by announcing the results of an interim analysis of the phase 3 clinical trial that the self-developed COVID-19 vaccine candidate ‘mRNA-1273’ has a prevention effect of COVID-19 of 94.5%. The price of the inoculation was set at $ 32-37 (35,700-41,300 won).
The price for ‘BNT162b2’, a candidate vaccine developed by Pfizer with German Bioentech, is $ 19.50 (21,700 won) per dose. On the 18th, Pfizer announced that the immunological effect of the vaccine was 95% as a result of the final phase 3 clinical trial of the vaccine.
According to the Daily Washington Post (WP), both the Modena and Pfizer vaccines require a total of two doses. The Modena vaccine is given again 4 weeks after the first vaccination and the Pfizer vaccine is given every 3 weeks.
The pharmaceutical company that set the highest price for the vaccines was Sinoparm from China. Sinoparm suggested that the cost of the Corona 19 vaccine being developed is $ 72.50 per dose. Sinopam is in phase 3 clinical trials for the vaccine.
According to the World Association for Vaccine Immunity, more than 60% of the world’s population must be vaccinated to obtain herd immunity. Population immunity refers to a condition in which the majority of the members of a specific group have immunity to viruses, so that disease transmission is not easily done.
The center said: “If there are many people who have difficulty paying for vaccination, the chances of ending COVID-19 will decrease. Therefore, various efforts are needed to solve the problem of access to vaccines and distribute them effectively. “.
Reporter Jeong Hye-jeong [email protected]
[ad_2]