[ad_1]
Global pharmaceutical companies like Pfizer are likely to receive approval for emergency use of the vaccine this year
The health authorities “are proceeding with purchase negotiations with foreign pharmaceutical companies … phase of the contract”
Government “Secure enough purchase lines and large quantities even with a large amount of upfront payment”
“Insuring 30 million people in a year through international advisory bodies and global pharmaceutical companies”
[앵커]
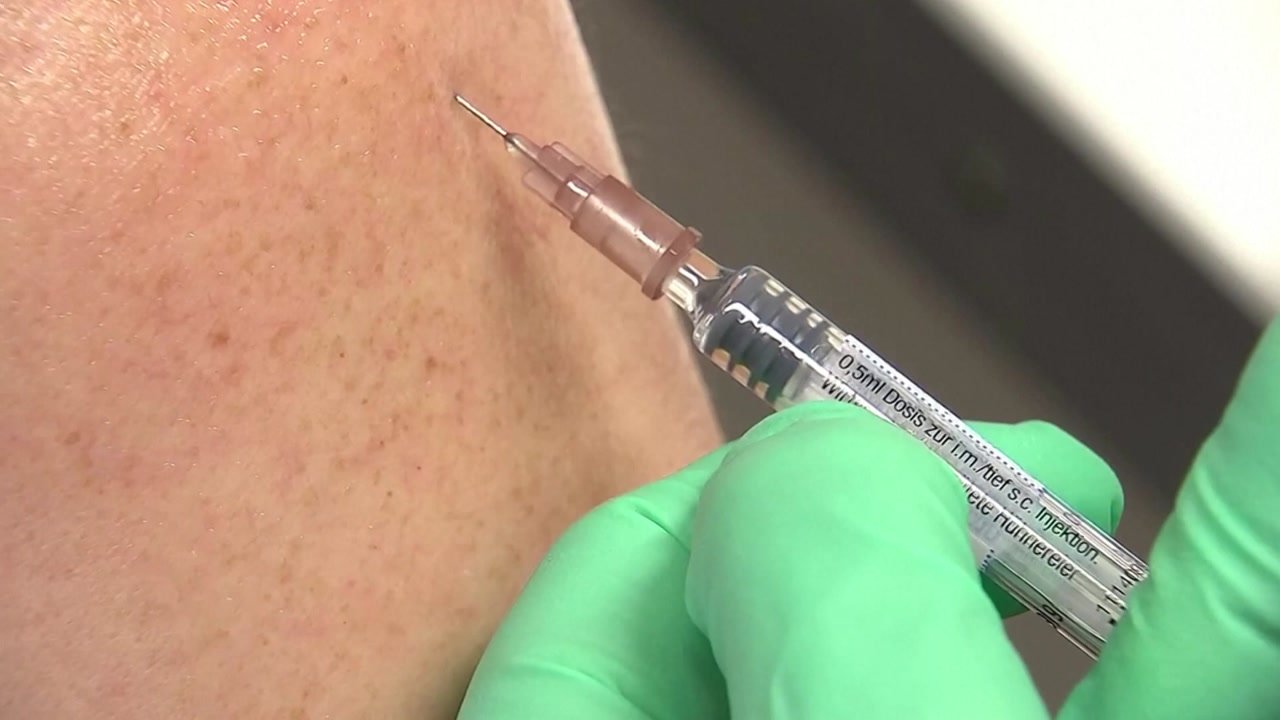
The government announced that it is negotiating with global pharmaceutical companies with the goal of securing a COVID-19 vaccine that can inoculate 60% of the total population or 30 million people within this year. After securing the vaccine, we plan to bring the vaccine as soon as possible through special import procedures. Reporter Park Hong-gu about the report. Currently, among global pharmaceutical companies, only four companies, including Pfizer and Modena, have entered the phase 3 clinical trial phase of COVID-19 vaccine development. They are expected to present the interim results of the phase 3 clinical trial to US health authorities later this month and receive urgent approval for use later this year. Health officials say they are currently negotiating purchases with some of them and are in the process of drafting contracts. The government plans to try to secure enough purchase lines and large quantities, even if some companies ultimately fail to develop vaccines and make upfront payments. As we have already insured 10 million vaccines through the International Vaccine Supply Council, we plan to insure 20 million people this year through individual negotiations with global pharmaceutical companies. A vaccine introduction advisory committee was also formed to discuss vaccine safety and protection, and the operation began in earnest. The import period is expected to be drastically shortened by applying a special import procedure for the importation of corona vaccine for the first time among infectious disease vaccines. The route is expected to be similar to remdesivir, a treatment for seriously ill patients with Corona 19, with the product being introduced first and then official approval reviewed. There is a precedent that the Ministry of Food and Pharmaceutical Safety formed an independent advisory body in June to allow the special importation of remdesivir. The government has announced that it will establish a vaccination plan calmly, taking into account the corona 19 epidemic in Korea and the vaccination situation in other countries along with efforts to secure vaccine quantities. This is YTN Park Hong-gu. ※ ‘Your report becomes news’ YTN awaits your valuable report.
[카카오톡] Search YTN to add a channel [전화] 02-398-8585 [메일] [email protected] [온라인 제보] www.ytn.co.kr