[ad_1]

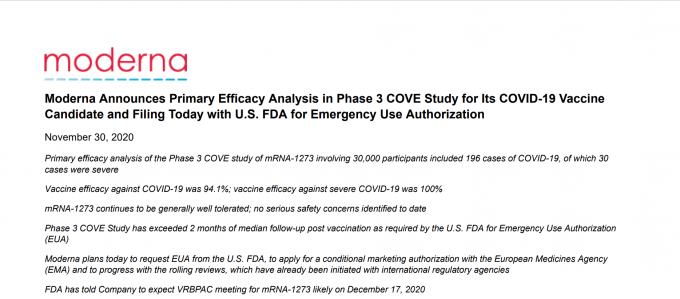
The American biotechnology company Moderna announced on its home page on the morning of the 30th (local time) that its new vaccine against coronavirus infection (Corona 19) has a preventive effect of 94.1% and that it is requesting the approval of emergency use by the United States Food and Drug Administration (FDA). Capture home or modern page
The American biotechnology company Modena announced the results of the phase 3 clinical analysis of the new coronavirus infection (COVID-19, Corona 19) on the morning of the 30th (local time), and today it requests the approval of emergency use from the United States Food and Drug Administration (FDA). Revealed.
Modena announced on this day’s website that it has begun procedures for the emergency use of Corona 19 vaccine in the United States. In an announcement that day, Modena also announced that it has applied for emergency use approval of the vaccine from the European Medicines Agency (EMA), and it is highly likely that the supply of Corona 19 vaccine from Modena will accelerate in Europe.
According to Modena’s announcement, the final analysis of the phase 3 clinical trial in which mRNA-1273, the company’s Corona 19 vaccine, was administered to 30,000 people showed that the prevention rate was 94.1%. This is similar to the preliminary analysis of the phase 3 clinical trial announced on day 16, which says that the effect of the vaccine is 94.5%.
Of the 30,000 participants in the phase 3 clinical trial, half of the 15,000 received the Corona 19 vaccine and the other half received a placebo (a dummy drug). Of the 15,000 people who received the Corona 19 vaccine, 11 confirmed cases of Corona 19, and of the 15,000 people who received placebo, 185 were infected with Corona 19. Of the 196 confirmed cases of Corona 19, 186 came out of the placebo group and the Vaccine prevention rate was analyzed at 94.1%.
In particular, Modena emphasized that of the 196 confirmed cases, 30 seriously ill patients came from the placebo group, and none of the Modena Corona 19 vaccine patients were seriously ill. One in 30 seriously ill patients died. Modena said: “The vaccine is 100% effective in preventing the outbreak of severe patients with corona19”.
Thal Sax Modena, Medical Director, appeared on CNN that day and said: “When I checked this result on Saturday (5) night, my emotions became intense and tears were shed.” “I hope that the vaccine changes the pattern of the pandemic. I’m doing.”
“This is great news,” Dr. Paul Oppit, a member of the FDA’s Vaccine Advisory Committee, told CNN about the results of the Modena vaccine.
Sax’s medical director also explained that the company’s vaccines were consistently effective regardless of age, race or gender, and that no serious side effects occurred. Modena’s phase 3 clinical trial included 7,000 people over the age of 65 and 5,000 young adults with chronic diseases such as diabetes and heart disease. Blacks, Asians, and ethnic minorities accounted for 37%, and the racial makeup was also diverse. The side effects of vaccination reported by Modena so far are fatigue, muscle pain, headache, and pain at the injection site, which are generally mild symptoms that can occur with vaccination.
In the case of the Pfizer vaccine, which applied to the FDA for the urgent use of Corona 19 vaccine for the first time before Modena, there were seriously ill patients among participants who received the vaccine in a phase 3 clinical trial.
US Health and Welfare Minister Alix Aza appeared on CBS’s “This Morning” the same day and said “there will be two vaccines (Pfizer and Modena) available to Americans before Christmas.”
It is reported that the FDA will finalize the emergency use approval review for Pfizer’s Corona 19 vaccine on the 10th. Modena is expected to be tried on the 17th, a week later. Following FDA approval, the Centers for Disease Control and Prevention (CDC) Advisory Committee announces priorities for vaccination recommendations in one to two days. “The main objective of the federal government is to guarantee a fluid supply of vaccines,” Aza said. “The state will decide who will get the vaccine first, referencing the CDC’s recommendations.”
Meanwhile, Europe also announced that the EMA started a continuous review of the vaccine shortly after the announcement of the intermediate results of the phase 3 clinical trial of the Modena vaccine on 16 last month (local time), which is expected to accelerate approval of the vaccine. However, apart from EMA approval, the vaccine supply schedule by country is likely to be different.
The British Daily Guardian said that the British government previously purchased 7 million doses (one dose) of the modder vaccine on the 30th (local time), and bought an additional 5 million doses last month and 2 million doses last week, but by the end of next March. It was predicted that it would be difficult to supply modders or vaccines to the UK.
As part of the Trump administration’s ‘Operation Warp Speed’, Modena developed a Corona 19 vaccine worth $ 2.48 billion (approximately 2,746.8 billion won), thus providing the vaccine to the United States first. Moderna expects to supply 20 million doses to the United States by the end of this year, produce between 500 million and 1 billion doses next year, and supply it to the world.
[ad_2]