
[ad_1]
The coronavirus pandemic, now “officially” in its second phase, is testing the resilience of groups around the world, waiting for the vaccine for salvation.
The resumption of confinement measures is already causing serious problems in the economy, while the rapid increase in deaths “generates” shocking images.
Professor Elias Mosialos, who told iefimerida.gr that Greece already has four-digit cases, previously published a Wall Street Journal “map”, which lists the 9 vaccines that have the potential to save Earth’s population from coronavirus.
Elias Mosialos’ post with the “map” of advanced vaccines (“click” to enlarge):
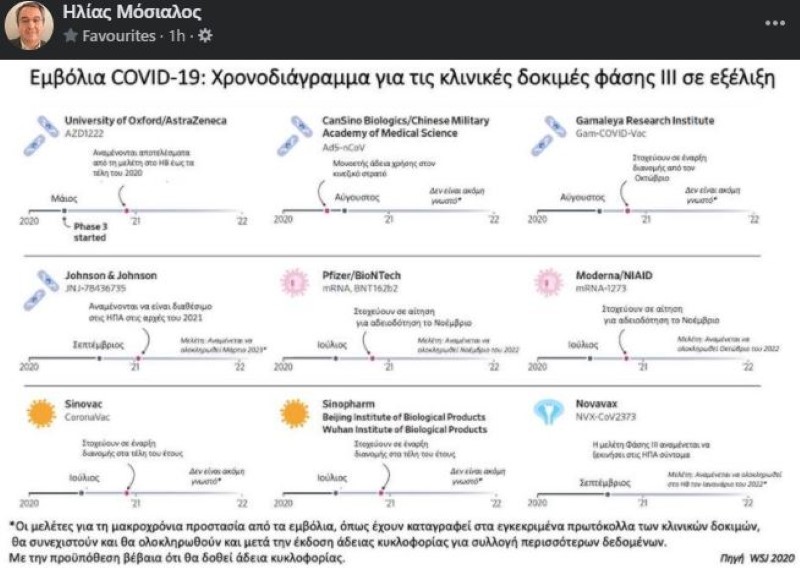
The 9 vaccines that can save the planet from coronavirus
The following is a list of vaccines that aim to “eradicate” the threat of coronavirus:
1) The Oxford vaccine and AstraZeneca
The Oxford University-AstraZeneca partnership vaccine has been in the third phase of testing since May. This is the only vaccine for which final test results are likely to be released in late 2020. Today, encouraging data on this vaccine and the elderly were announced. AstraZeneca claims that it can prepare up to 2 billion doses of the vaccine and that 50% or 1 billion can be distributed by 2020.
2) The CanSino vaccine
The Chinese CanSino, according to the Wall Street Journal, has developed a vaccine based on the common cold coronavirus. He has already received a one-year license to use the vaccine in the Chinese military. No further details have been provided for the final completion of the studies. Production capacity is estimated at 100 to 200 million doses (as of 2021).
3) The “hasty” candidacy of the Russians
The Gamaleya Research Institute (Russia) vaccine is, according to the Russian government, at a very advanced stage. The team aims to begin distribution in October, although Phase III studies have not been completed. The Russians estimate that they can produce 500 million doses of the vaccine a year.
4) The Johnson & Johnson vaccine
The Johnson & Johnson vaccine is expected to be available in early 2021. However, the study will continue and is expected to be completed in March 2023. Ongoing trials are underway in 60,000 people. Some early and encouraging studies have shown a strong immune response. In terms of production, according to the Wall Street Journal, it is estimated to reach 1 billion doses of vaccine by the end of 2021. These doses include 100 million doses for the US and 30 million doses for the UK.
5) The Giants Association (Pfizer / BioNTech)
The Pfizer / BioNTech partnership aims to apply for a license in November. The study of this vaccine is expected to be completed in November 2022. The ongoing trial involves 30,000 people. The Wall Street Journal reports that, if approved, there could be 100 million doses of the vaccine by the end of 2020 and up to 1.3 billion doses by the end of 2021.
6) The Modern vaccine
The Moderna / NIAID vaccine is scheduled for licensing in November. Trials involving 30,000 people are ongoing and the study, including in the case of licensing, is expected to continue through October 2022. Some early positive data showed antibody production. It should be noted that, according to the Wall Street Journal, a vaccine with the technology used by Moderna to fight a disease has never been approved. Estimated Manufacturing Capacity: 500 million with 1 billion doses of vaccine as of 2021.
7) Η Sinovac
China’s Sinovac is conducting major trials in Brazil and aims to begin distributing the vaccine in late 2020. It is not known how long the study will last. Early research has shown that it is safe. An agreement has already been reached with India on the distribution of 250 million vaccines. Sinovac’s team estimates that it will be able to produce 300 million doses a year in Beijing.
8) Sinopharm’s double candidacy
Sinopharm’s Chinese team, made up of scientists from Beijing and Wuhan, and essentially two vaccines, expects to launch the vaccine later this year. It is not known when the study will be completed. In case of approval, this group has foreseen the possibility of preparing 220 million annual quotas.
9) The two doses of Novavax
The phase III study of the Novavax vaccine will soon begin in the US The study is expected to be completed in the UK in January 2020. This vaccine is given in two doses 21 days apart. Estimated production capacity: 100 million US installments with distribution end of 2020 and 2 billion annual installments for global distribution in 2021.
The footnote to the advanced vaccines “map” published by Elias Mosialos highlights that “studies on long-term protection against vaccines, as recorded in approved clinical trial protocols, will continue and be completed after issuance of a marketing authorization for the collection of more data. Provided, of course, that a marketing authorization is granted. “
[ad_2]