[ad_1]
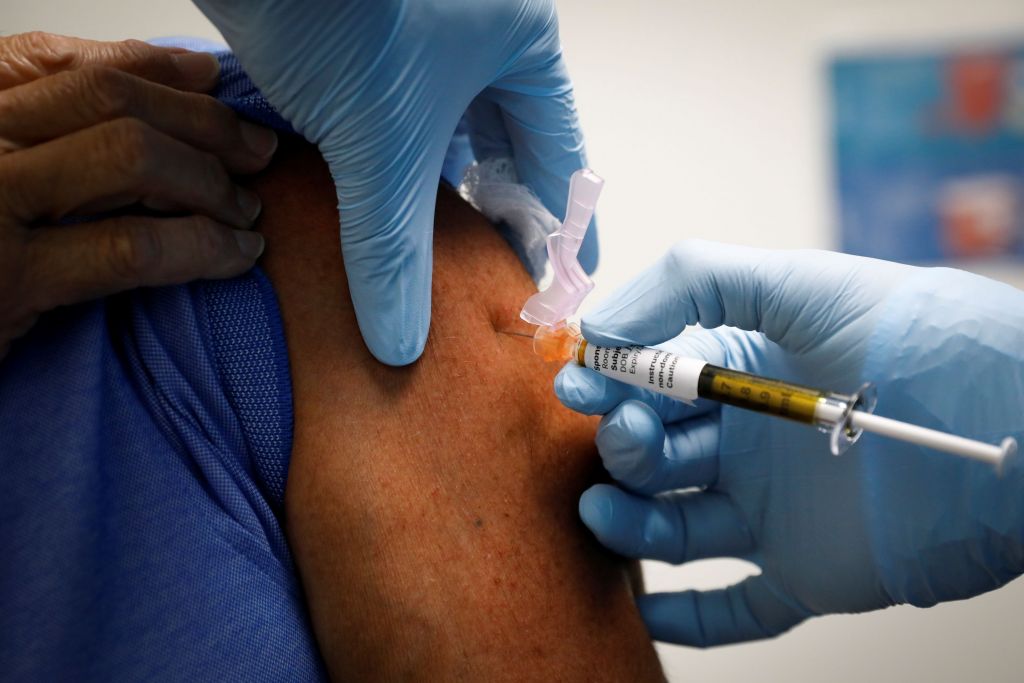
In the wake of the encouraging announcements about at least three candidate Covid-19 vaccines, European officials estimate that the long-awaited emergency clearance could arrive before the end of the year.
“The first European citizens can be vaccinated before the end of December,” European Commission President Ursula von der Leyen said in a speech to the European Parliament on Thursday.
For the first time, the head of the Commission spoke of “light at the end of the tunnel.”

Commission President urges European governments to prepare for mass vaccination (Reuters)
“The European Commission has now secured contracts with six companies. The first European citizens can get vaccinated before the end of December.” Finally there is light at the end of the tunnel, “he said.
Contracts with pharmaceutical companies
Von der Leyen referred to contracts signed by the EU with six manufacturers: Pfizer / BioNTech, AstraZeneca, Moderna, Sanofi / GSK, CureVac and Jansssen.
The top three companies have already submitted efficacy data, according to which candidate vaccines prevent 70 to 95 percent of coronavirus cases.
What is pending is approval of the vaccines by the European Medicines Agency (EMA), which began examining safety and efficacy data even before pharmaceutical companies submitted applications for approval.
The Commission’s final green light
EMA, based in Amsterdam, is responsible for the licensing and control of medicines in the European Union. The final green light, however, is given by the European Commission.
The service chief estimated on Wednesday that the approval process could be completed within a year.
“If all goes well, we have high hopes of having a positive opinion before Christmas,” EMA CEO Emer Cook told RTE.
On Tuesday, the Irish Independent newspaper published an interview with Cook, according to which the agency’s goal is to follow a schedule similar to that of the US FDA, which is expected to provide an opinion on the Pfizer / BionTech vaccine to mid december.
“What we are hearing at the moment are the results of clinical trials, and they are very positive.” But until we look at the clear data and find that they have ‘as the chart says’, we cannot recommend their use to the European public. ” Cook said.
Uncertainty
However, Coreper does not rule out the possibility that the approval of the first vaccines will arrive in early 2021. “At this stage, it is difficult to accurately predict the licensing times of vaccines, since we do not yet have all the data and results of ongoing evaluations. “It refers to an email sent by Coreper to the French Agency.
In addition to licensing, there is the question of organizing vaccination programs for the 448 million Europeans.
“Vaccines are important, but it’s the vaccines that count,” von der Leyen said.
“Member States must prepare now […], you must prepare the chamber for the development of hundreds of millions of doses of vaccines. “Because that’s what our exit from the pandemic will mean,” he said.
 at google news and be the first to know all the news
at google news and be the first to know all the news
[ad_2]