[ad_1]
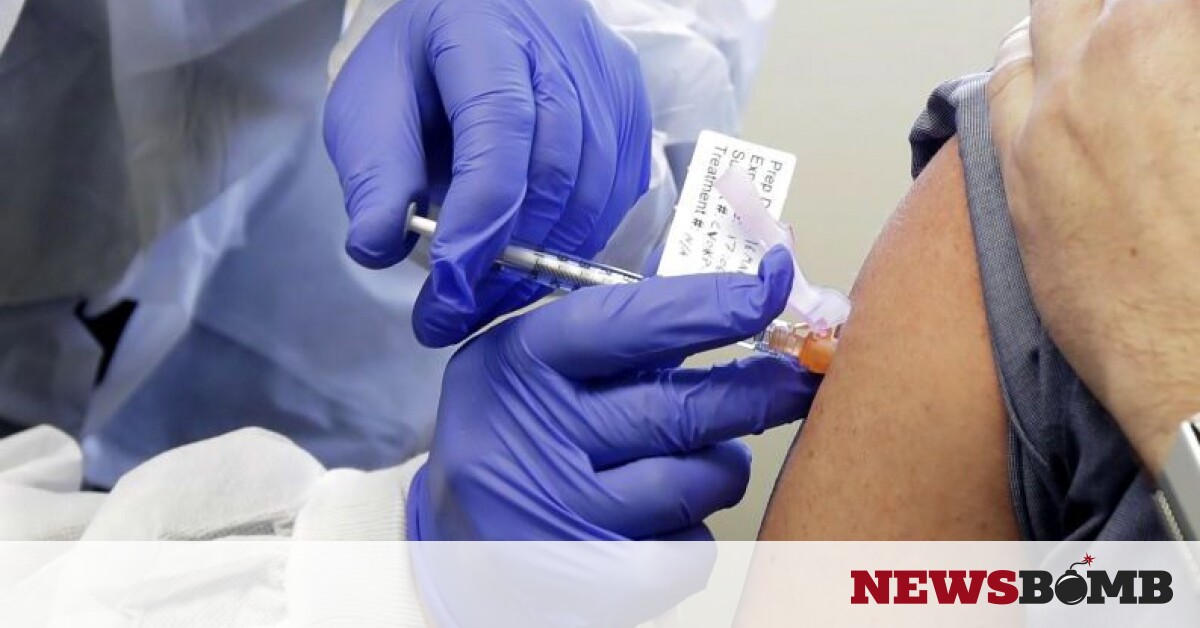
Vaccine for coronavirus: FDA Approves Emergency License and Modern Vaccine After Pfizer.
The second vaccine that obtained a license in the United States became that of Moderna. Following the coronavirus vaccine developed by Pfizer / BioNTech, another has received FDA approval and is expected to be available to citizens soon.
According to Bloomberg, the votes in the competent committee were 20-0 in favor of the vaccine. According to the report, the committee ruled that the benefits of the vaccine far outweighed the risks of early approval. In the next few days there will be official permission to Modern with the vaccine made available to the public.
We remind you that your vaccine Modern It is expected to be examined by the European Medicines Agency a week before.
This company submitted additional data earlier than expected and Coreper will meet on January 6, six days before the scheduled date, so you can decide whether to approve the vaccine. If that happens, it will be the second vaccine approved by the European Union.
News from Greece and the world, as it happens, on Newsbomb.gr.
Read also:
Newsbomb Revelation: This is how the cocaine circuit worked. The … beers, coffees and 235 customers