[ad_1]
A historic day for her Britain, but also for all humanity, it evolved on Tuesday (12/8), when the vaccines for covid-19 began, a fact that marks the countdown to return to normality, a moment that is expected to be a few months later.
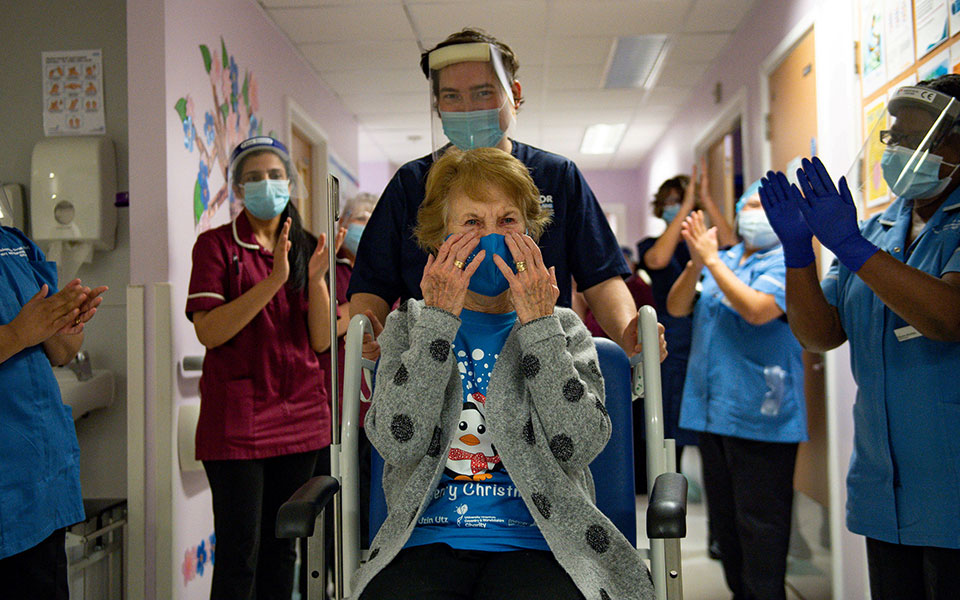
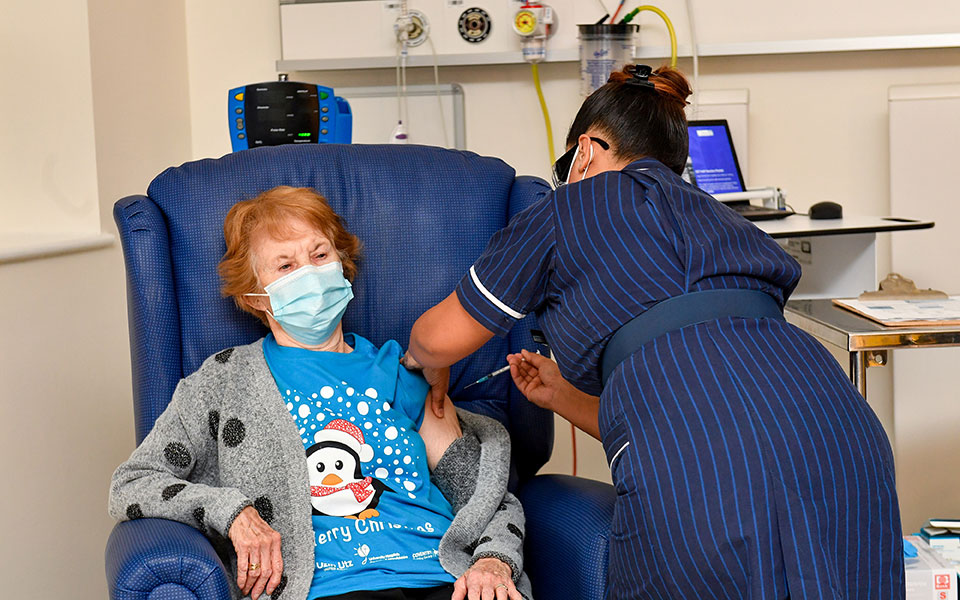
The first vaccine was given to the 90-year-old. Margaret Keenan, a few days before his birthday. The elderly woman, who was applauded by the nursing staff upon her departure, received the first of the 800,000 Pfizer / BioNTech vaccine doses to be administered to physicians, nurses, vulnerable groups and the elderly in the coming days. They are still waiting for the end of the month. 4 million doses in Great Britain.
“I cannot thank more to the Health System staff who took great care of me. My advice for the vaccine is “go for it.” “If I can do it in my 90s, you can too.”
On Tuesday, according to sources cited by the BBC, they took place thousands of vaccines across the country.




The series of vaccinations
The order in which the British population will be vaccinated, according to the schedule of the Joint Committee on Vaccination and Immunology for Phase 1 is as follows: (In parentheses the population number corresponding to this category).
1. Inmates in nursing homes (425,000) and their nurses (1.5 million)
2. All those over 80 years of age (3.3 million) and first-line hospitals (1.5 million)
3. Anyone older than 75 years (2.2 million)
4. All people over 70 years of age and those considered clinically particularly vulnerable (3.3 million)
5. All citizens over 65 (65 million)
6. Citizens aged 16 to 64 with underlying illnesses
7. People over 60 years old (3.7 million)
8. People over 55 years old (4.3 million)
9. All citizens over 50 (4.7 million in mid-January)
What will happen to people aged 16 to 49? Unless they have an underlying disease, people in this age group will not be vaccinated in Phase 1.
What will happen to the children: Most children under the age of 16 will not be vaccinated, according to the scientific community’s findings. However, children at high risk of exposure will be vaccinated.
What is provided to pregnant women: In the absence of safety data, vaccination is not allowed for any woman who is already pregnant or expecting to become pregnant during the next three months. Mothers who breastfeed are also excluded (they are not allowed to get vaccinated).

The UK is the first country in the world to start using the Pfizer vaccine after regulators approved its use last week.
The British Minister of Health, Matt hancockHe stressed that there is “light at the end of the tunnel”, and noted that “the counterattack against our common enemy, the coronavirus, has begun.”
It’s worth noting that Britain announced 12,282 new cases and 616 deaths on Tuesday.
The Pfizer / BioNTech vaccine is stored at a temperature of at least -70 degrees Celsius and lasts for five days at normal freezing temperatures. For this reason, the Ministry of Health stressed that the vaccine will be administered first to 70 hospitals, noting that it will take a few hours to thaw each vaccine and prepare it for use.


Europe
At the same time, the director of BioNech, Ugur SahinHe estimates that the vaccine will be approved in the EU at the end of December. You have not yet been vaccinated because there is no relevant license for the vaccine from your company. “We still don’t have a vaccine license in Germany.” But once it’s approved, of course I’d like to do it, “he said. Read
United States
In a parallel evolution, the Food and Drug Administration (FDA) The United States is one step ahead of its approval the Pfizer vaccine, since the documents published today certify that the preparation is safe and effective.
FDA scientists conducted their own extensive investigation and confirmed Pfizer’s assessment, that is, their vaccine was 95% effective. They also confirmed that its short-term side effects (pain in extremities, exhaustion, headaches, muscle aches) are actually tolerable, while they cease after two days. Read in detail

What happens when someone receives the vaccine?
The vaccine, which has been developed using the new “messenger RNA” technology, is administered by injection into the arm. Immunization is done in two doses three weeks apart, and as tests have shown it protects up to 95% of those who have done so from contracting COVID-19.
Pfizer has stated that side effects in those who participated in the trials were predominant mild to moderate and regressed rapidly. The most serious side effects occurred after the second dose: fatigue in 3.8% of volunteers and headache in 2%. Older participants, however, seemed to report fewer and milder side effects of this type.
What type of protection does the vaccine provide?
The vaccine begins preventing COVID-19 infection seven days after the second dose, which comes about a month after the first.
Clinical trials have not yet been designed to determine whether an immunized person can transmit the virus to another person. Some vaccines, such as hepatitis A vaccines, provide this type of protection, also known as “sterile immunity,” while others do not.
During the trials, COVID-19 vaccine manufacturers focused on whether the vaccine would prevent someone from getting sick.
It will also take even more months before it becomes clear how long the vaccine protects someone from becoming infected.
“Until then, it’s best to avoid bars and other crowded gatherings,” said Dr. Anita Set, an infectious disease specialist at the Johns Hopkins Bloomberg School of Public Health.
Does vaccination mean returning to normal life?
Since there is no evidence that immunization inhibits transmission of the virus and no vaccine is 100% effective, scientists are calling for constant vigilance by wearing a mask, washing hands, and keeping distance.
“The vaccine, like all vaccines, may seem to work very well in some subcategories of patients, but not so well in others. Does this mean that we are free to fly or invite 30 people to our home? Probably not.” , said. Dr. Michelle Baron, Director of Infection Prevention at UC Health, Colorado.