[ad_1]
Rapid antigen tests for the SARS-Cov-2 virus are intended to help ensure greater safety and normalcy, especially in nursing homes and schools. However, they are not a panacea.
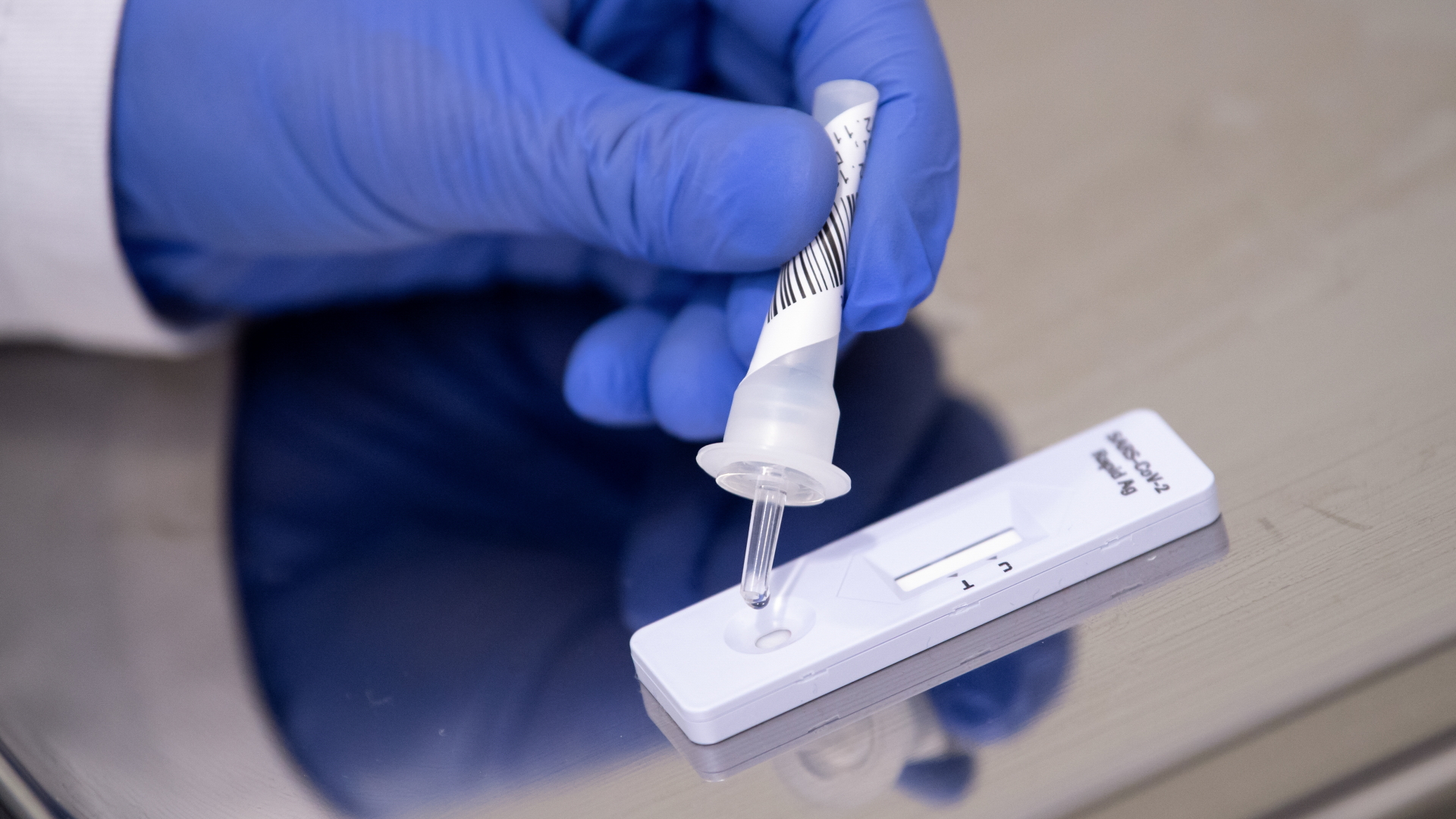
For a rapid antigen test, a sample is taken deep in the nose or throat. If it contains protein fragments of the virus, a positive test result is displayed.
A Covid 19 infection develops in three phases: First, the actual infection occurs. After that, the affected person is highly infectious, but does not have to show any symptoms of the disease. Ultimately, it may show symptoms and barely produce viruses, but it can still infect other people.
Rapid tests detect viruses in the most important stage for the spread of the disease. A positive result must be verified by a PCR test; a negative test does not guarantee that the subject is not contagious.
Rapid tests are significantly cheaper than PCR tests. It is not necessary to send them to a laboratory, but deliver the result on site in a few minutes. This eliminates sources of error such as transportation problems or confusion, and labs are relieved.
Under a new regulation, daycare centers and schools or their providers can obtain rapid antigen tests. Before that, this was only officially possible for doctors and hospitals. Opinions differ as to whether tests can and should be performed by non-professionals on the test subjects themselves: errors in removal or processing can falsify the result.
Physicians in private practice caution against use by non-professional physicians. This increases the risk of incorrect negative results and therefore the risk of “super spreaders” that are then “weighed with misleading security,” explained the NAV-Virchowbund. In the opinion of experts, testing should only be performed under sterile conditions, which are not available in most settings.
Therefore, the Robert Koch Institute (RKI) recommends the use of hygiene agents in nurseries and schools for testing, which are trained for the application. The Thuringian Ministry of Health and the Lower Saxony Medical Association recommend that rapid tests be used only by medical specialists or with prior training. Therefore, the associations of teachers, patients and caregivers demand support from external forces.
According to Health Minister Jens Spahn, Germany has secured more than 60 million rapid corona tests per month through guarantee contracts with large producers and suppliers. However, it’s daunting hopes in the WDR that they will be available in every nursing home and nursing home by Christmas – deliveries are still not enough everywhere, so Spahn. But that will improve step by step.
Rapid tests do not have the high precision of PCR tests. According to the Paul Ehrlich Institute (PEI), the Federal Institute for Vaccines and Biomedical Medicines, they are even “orders of magnitude” lower. Virologist Christian Drosten de la Charité cautions that this can lead to many false positive results in mass tests in the current infection situation.
The PEI has now developed minimum criteria for a possible approval of antigen tests. Consequently, the sensitivity must be 80 and the specificity 97 percent, that is, 80 out of 100 positive samples must be recognized as such and only three out of 100 healthy test people are incorrectly shown as infected. However, these requirements are not yet binding.
Only some of the more than 200 tests offered in Germany so far have been independently verified. In a preliminary study by virologist Drosten, the specificity of seven products tested was between 88.24 and 100 percent.
For in vitro diagnostics, which also include rapid antigen tests, there is no obligation to obtain approval or independent testing. Until this is implemented as planned, users should trust the information provided by the manufacturer.