[ad_1]
A new variant of SARS-CoV-2, the virus that causes COVID-19, is believed to be driving further transmission of the disease in parts of the UK. The government has placed some regions, including London, under new stricter coronavirus restrictions, known as Level 4. People in Level 4 areas will not be able to meet anyone outside their home for Christmas, while those in the rest of the country they can only meet. the same day of Christmas.
Boris Johnson, the prime minister, and his top scientific advisers said the new variant could increase COVID-19 transmission by up to 70% and increase the R or reproduction number by 0.4%.
What is the significance of this new discovery? The Conversation asked Lucy van Dorp, a microbial genomics researcher and pathogen evolution expert, some key questions about what we know right now.

Toby Melville / PA
What do we know about this new variant?
The new UK variant, known as VUI – 202012/01 or B.1.1.7 lineage, was first identified in the county of Kent on 20 September. Matt Hancock, the health secretary, first announced the existence of the variant on December 14; it was subsequently confirmed by Public Health England and the UK COVID-19 sequencing consortium.
The variant carries 14 defining mutations, including seven in the spike protein, the protein that mediates entry of the virus into human cells. This is a relatively large number of changes compared to the many variants we have in circulation globally.
To date, the genetic profiles, or genomes, of this variant have been sequenced and shared largely from the UK, but include some in Denmark and two cases in Australia. There have also been reports of a case in the Netherlands. All of these countries have extensive genome sequencing efforts and it is quite possible that these observations do not reflect the true distribution of this variant of the virus, which could exist undetected elsewhere. We will know more as more genomes are generated and shared.
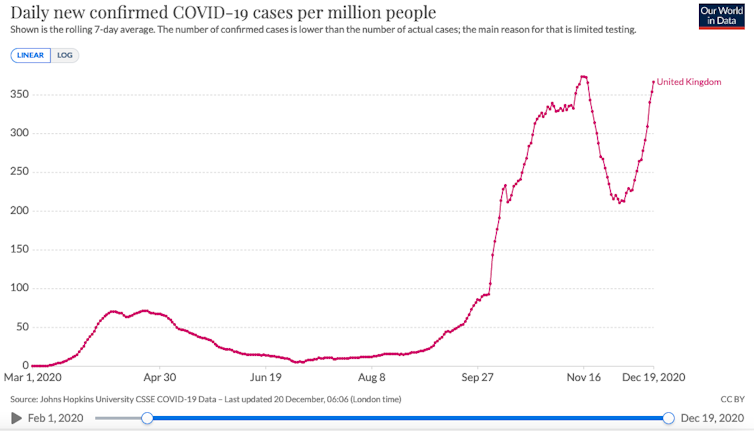
Thanks to data sharing efforts, genomic surveillance and COVID-19 test results in the UK, it appears that this variant is now starting to dominate existing versions of the virus and may be responsible for a growing proportion of cases in some parts. of the country, particularly in regions where we also have rapidly expanding case numbers.
It is always very difficult to untangle cause and effect in these cases. For example, increases in the occurrence of certain mutations may be due to the viral lineages that carry them increase in frequency only because they are those that are present in an area where transmission is high, for example, due to human activities or choice of interventions.
Although this is still a possibility, there are sufficiently clear observations thus far for this variant to warrant very careful characterization, surveillance, and interventions to curb transmission.
Is more dangerous?
Chris Whitty, the medical director, clearly stated that to date there was no evidence that this variant alters the severity of the disease, either in terms of mortality or the severity of COVID-19 cases for those infected. Work is underway to confirm this.
How do virus mutations occur?
Mutations are a natural part of the evolution of the virus. In the case of SARS-CoV-2, these mutations can arise due to random errors during the replication of the virus, be induced by antiviral proteins within infected people, or through a genetic mix, known as recombination. Although no signs of recombination are currently detected in SARS-CoV-2.
Most viral mutations are expected to have no impact. For example, when our team evaluated individual mutation replacements in more than 50,000 genomes from the first wave of the pandemic, we did not detect any that significantly altered viral fitness – the virus’s ability to survive and reproduce.
However, once in a while a mutation, or in this case a particular combination of mutations, can get lucky and give the virus a new advantage. Viruses carrying these combinations of mutations can be increased in frequency by natural selection given the appropriate epidemiological setting.
Design_Cells / Shutterstock
Where did the variant come from?
Right now, we don’t know. To date, scientists have not identified any closely related viruses to support the theory that the variant had been introduced from abroad. The observed patterns of mutations further support a prolonged period of adaptive evolution, probably in the UK, based on current data.
Similar mutation patterns to these have been observed in the evolution of SARS-CoV-2 in chronically infected patients with weaker immune systems. The current hypothesis is that such a chronic infection scenario, in a single patient, may have played a role in the origin of this variant. This will continue to be investigated.
How many variations of SARS-CoV-2 have we found?
There are many thousands of SARS-CoV-2 lineages that differ on average only by a small number of defining mutations. It remains true that the SARS-CoV-2 currently in global circulation has little genomic diversity. The subtleties in the mutations carried in different lineages can, however, be very useful in reconstructing transmission patterns.
As an example, early work in the pandemic used lineage assignments to identify at least 1,000 introductions of SARS-CoV-2 in the UK.
Why is this different?
It is important to note that many of the mutations that define the UK variant have been observed in SARS-CoV-2 before and sometimes even quite early in the pandemic.
However, the UK variant or lineage is defined by an unusual number and combination of mutations. One of these mutations, N501Y, has previously been shown to increase the binding of the virus to receptors on our cells. N501Y was first sequenced in a virus in Brazil in April 2020 and is currently associated with a variant of SARS-CoV-2 as well. increasing in frequency in South Africa – an independent lineage from B.1.1.7 which is also cause for concern.
OurWorldInData, CC BY-SA
The particular deletions identified in the B.1.1.7 spike protein have appeared in many other virus lineages with increasing frequency and are also seen in chronic infections where they can alter antigenicity – recognition by immune antibodies. These deletions may also be associated with other mutations in the peak protein binding region of the coronavirus, including those seen in infections among farm mink and a mutation that has been shown to play a role in the virus’s ability to evade the system. immunological in humans. B.1.1.7 also harbors a truncated ORF8 gene, with deletions in this region previously associated with decreased severity of disease.
The functional effect of these mutations and deletions, particularly when found in the combination reported in B.1.1.7, is yet to be determined. The high number of mutations and the recent increase in the prevalence of this particular variant, together with the biological relevance of some of the candidate mutations, emphasizes the need for an in-depth study.
What does this mean for the vaccine?
At the moment we do not know. Although we must be sure that the vaccines stimulate a broad antibody response to the entire peak protein, it is anticipated that their efficacy will not be significantly hampered by mutations. This is is already being tested.
However, there is mounting evidence that other species of seasonal coronavirus exhibit some ability to escape immunity for longer periods of time.
Therefore, it is conceivable that we will reach a point where we are required to update our COVID-19 vaccines, as we do for influenza, to reflect the variants in circulation at that time. It is too early to say if this will be the case now, but extensive genome sequencing, data sharing, and standardized variant reporting will be vital to inform these efforts.
[ad_2]