[ad_1]
Russia’s Direct Investment Fund announced the readiness of the developer of the Russian vaccine Sputnik V to produce the drug based on the capabilities of the Ukrainian pharmaceutical company Biolek following a meeting in which the People’s Deputy of the Opposition Platform participated – For Life and the Godfather of Russian President Vladimir Putin Viktor Medvedchuk. … The administration of “Biolek” stated that the company can only produce drugs registered in Ukraine.
On December 8, the Russian Direct Investment Fund (RDIF) published a press release on the meeting of the People’s Deputy of the Opposition Platform – For Life and the godfather of Russian President Vladimir Putin Viktor Medvedchuk with the head of the background Kirill Dmitriev and the director of the Russian Center for Epidemiology named after Gamaleya, in which the vaccine was developed. of the coronavirus, Alexander Gunzburg.
The Foundation said the meeting discussed the possibility of technology transfer and the launch of production in Ukraine of the Russian vaccine “Sputnik V”.
The RDIF Gamaleya Center is ready to produce the vaccine in Ukraine and submit documents for registration, the statement said.
The authors of the message wrote that the Kharkov pharmaceutical company “Biolek” had allegedly already launched the production of a finished form of the vaccine.
However, the Biolek company has denied this claim.
Biolek, as a national vaccine manufacturer, is technically capable of producing a coronavirus vaccine, but only if it has its state registration in Ukraine. At the moment, JSC Biolek has not signed any contracts, has not received any transfer technology for the production of the Sputnik V vaccine or any other coronavirus vaccine, and has never produced such vaccines on the company’s technology base “, said the company’s management.
Ukraine’s Ministry of Health knows nothing about the possibility of releasing “Sputnik V” in Ukraine, said the deputy director of the department, the country’s chief sanitary physician, Viktor Lyashko, in a comment to LB.ua.
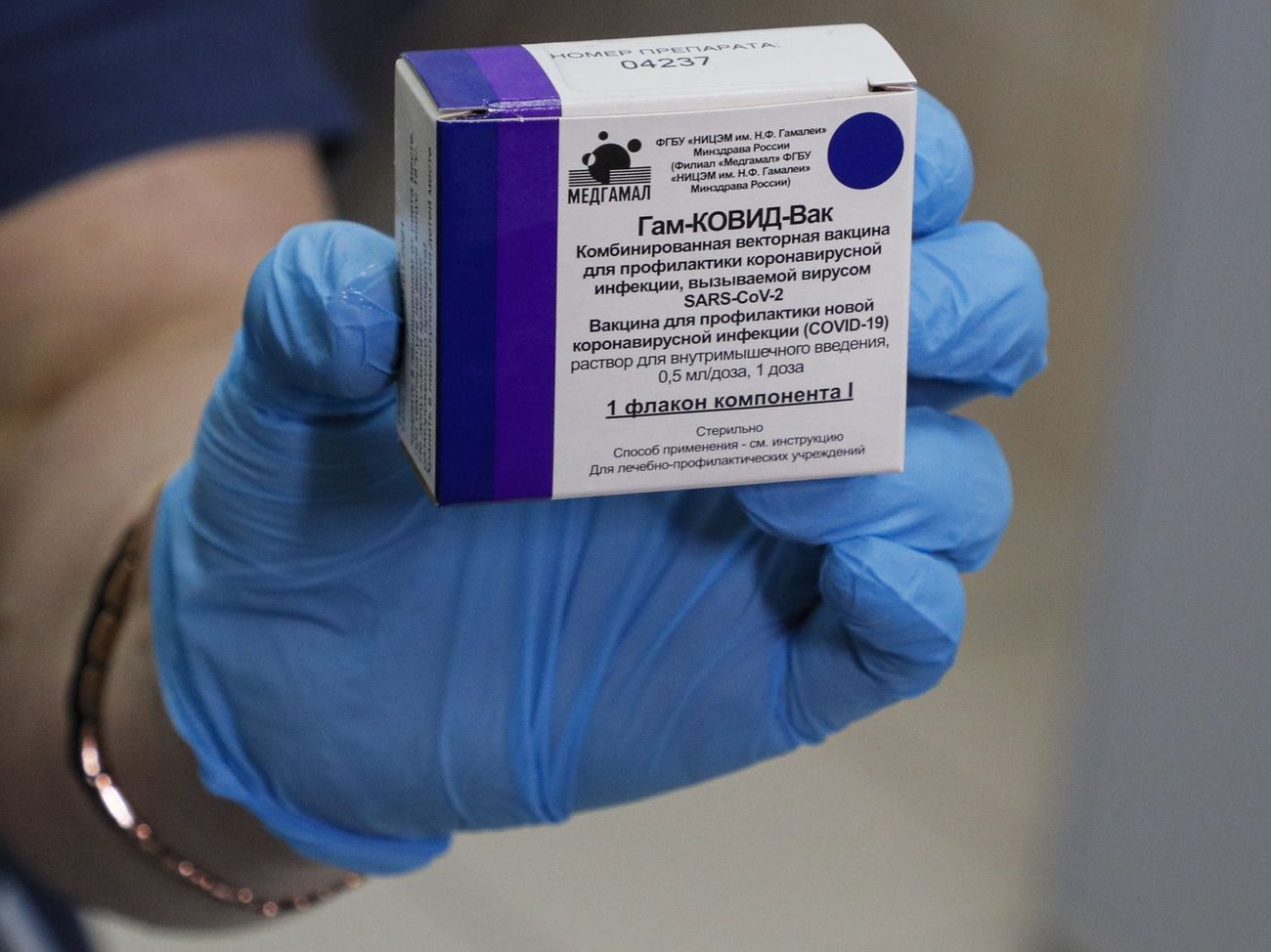
The registration of the “world’s first vaccine” against coronavirus infection – “Sputnik V” (“Gam-COVID-Vac”) – announced by Russian President Vladimir Putin on August 11. According to him, the vaccine is effective, forms stable immunity and has passed all tests. Putin said one of his daughters received two vaccinations and was feeling fine. At the same time, the Russian president himself was not vaccinated; As head of state, he cannot volunteer in a vaccine trial, explained Dmitry Peskov, the Russian leader’s press secretary.
The Russian vaccine was widely criticized as it was registered before the third phase of clinical trials. Phase 3 trials are critical to testing a vaccine’s readiness for widespread distribution, writes National Geographic.
Ukraine’s Health Minister Maxim Stepanov said there is no Russian vaccine against COVID-19.
“When they say they have registered a vaccine that has not passed the third stage [испытаний] – there is nothing to talk about. The third stage is the largest in which the vaccine is tested, even from the point of view of its safety, ”he explained on December 3.
The minister said Ukraine is negotiating with four COVID-19 vaccine manufacturers: three from the United States and one from China; they are already in their final stages. In addition, Kiev hopes to receive free vaccines as part of the global COVAX initiative.
“We can only use vaccines that have passed all the stages of clinical trials and are duly registered. Yes today [американская] Pfizer has passed all the stages of clinical trials, has announced all the results of these stages, the first, the second and the third, and it is registered, we can say: “We have a vaccine”, and the whole world is talking about it . And when they are at home [в России] vaccinate in parallel and go through the third stage, […] God knows what’s going on there. But they started getting vaccinated without completing the third stage of clinical trials, ”Stepanov emphasized.
Several Russian scientists have observed that the fast-track approach to vaccine registration is contrary to scientific and ethical standards for drug development.
The Russian vaccine is incomplete, according to the United States.
In September, the Ministry of Health of the Russian Federation stated that side effects were manifested in “about 14%” of the volunteers vaccinated with Sputnik V during the investigation. In October, Gunzburg reported side effects in 15% of trial participants. He said that “some” of the volunteers who participated in the trials contracted COVID-19.
[ad_2]