
[ad_1]
The Verkhovna Rada has supported the bill №4 314. The document will reduce the period of approval of clinical trials and registration of vaccines against coronavirus.
It was supported, reports channel 24. By the way, on December 2, the profile committee recommended to the Rada to support the bill.
Read also The coronavirus vaccination strategy will be ready next week, – Stepanov
So, according to the chairman of the Verkhovna Rada Committee on National Health, Medical Assistance and Health Insurance, Mikhail Radutsky, the passage of the bill will reduce the burden on the health system.
What the invoice provides:
- shorten the deadline for approving clinical trials and registering vaccines;
- extend the period of validity of the Law of Ukraine No. 539-IX, to similar provisions for drugs for the treatment of COVID-19 until the end of the quarantine established by the Cabinet of Ministers of Ukraine.
At what stage is preparation for vaccination against COVID-19 in Ukraine?
- Ukraine will receive around 8 million doses of coronavirus vaccine in the COVAX initiative. By December 7, according to the head of the Ministry of Health, all relevant documents will be ready in Ukraine.
- Ukraine has reached an agreement with Pfizer. The company expects delivery of the vaccine in early Q2 2021.
- COVAX is the mechanism for global access to COVID-19 vaccines. The initiative foresees that, in the first place, vaccines are carried out to people at risk. The initiative will provide the vaccine to 92 countries free of charge or below market price. Ukraine is also included in this list.
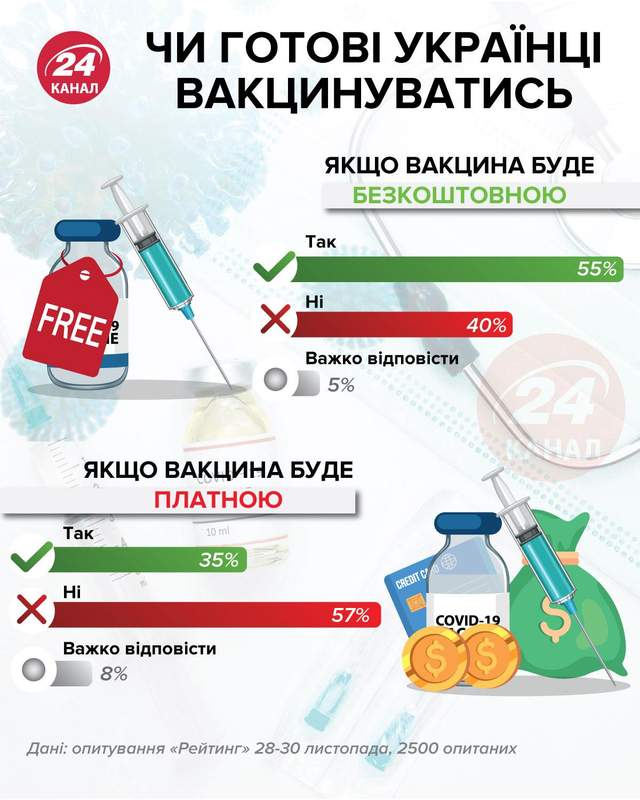
Are Ukrainians ready to get vaccinated? / 24 channel infographic