[ad_1]
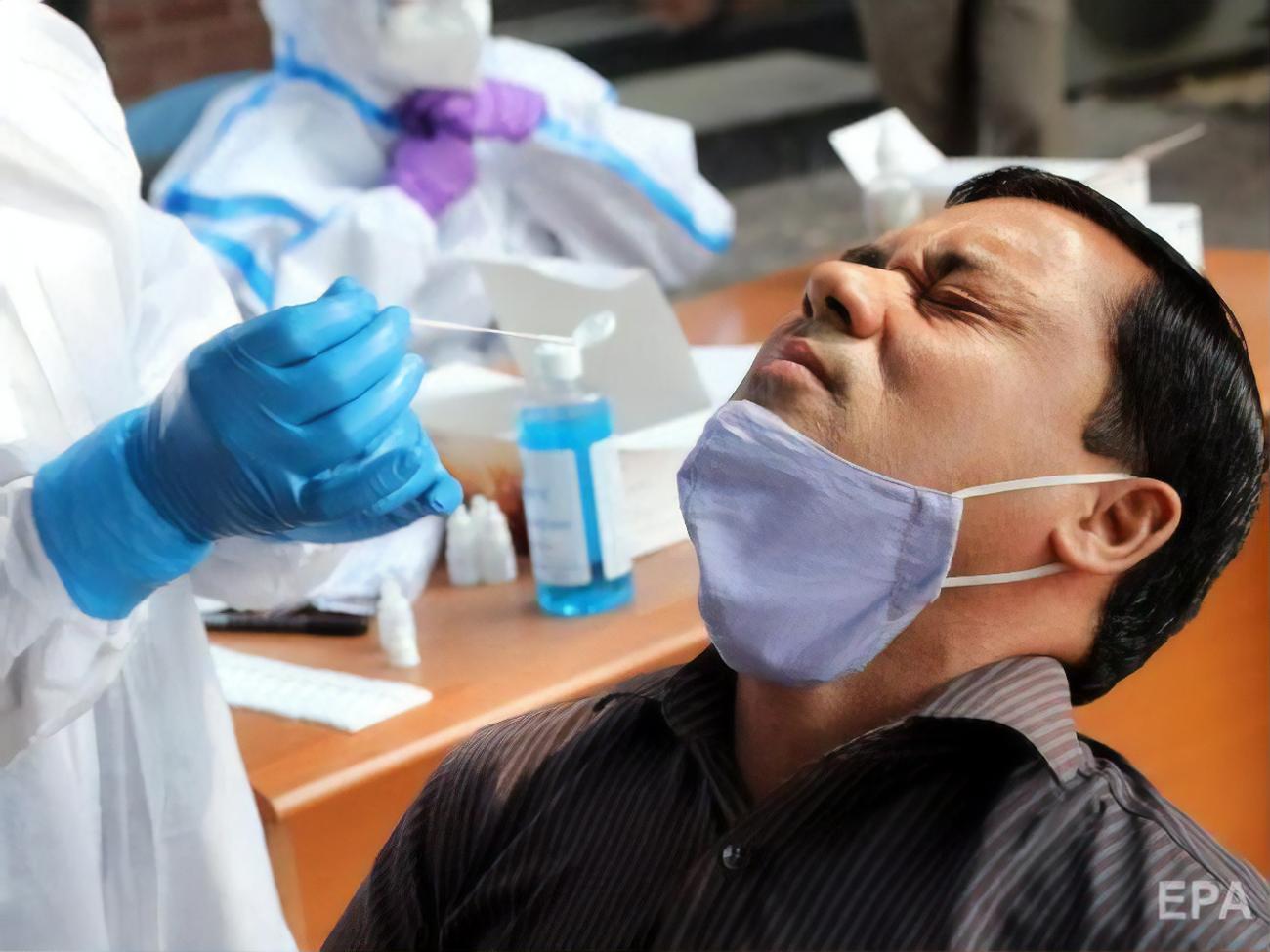
The FDA has approved the Lucira COVID-19 All-in-One Test Kit for home use with nasopharyngeal specimens from people 14 years of age and older.
On November 17, the FDA approved the first home test for a new coronavirus infection. The press service of the department reported.
“This new testing option is an important diagnostic advance in addressing the pandemic and reducing the burden of disease transmission in the population,” said Commissioner Stephen Hahn.
The Lucira COVID-19 All-in-One Test Kit has been approved for home use with nasopharyngeal specimens from individuals 14 years of age and older. The test is also approved for use in a healthcare facility. You must wait about 30 minutes to get the result.
Until now, the test can only be purchased with a prescription.
The United States remains the world leader in the spread of the coronavirus. As of today, the number of infected people has reached 11,357,244, 248,666 people have died, 4,293,640 have been cured.
In the world, 55,578,685 people have been infected with the coronavirus infection, 1,337,559 people have died from COVID-19, and 35,739,244 have recovered.
An outbreak of coronavirus infection began in late 2019 in China. March 11, 2020 World Health Organization declared the spread of the coronavirus a pandemic.
[ad_2]