[ad_1]
Just this week, we had the first promising report of a drug that appears to improve the recovery time of patients with COVID-19. Immediately after that announcement, a scientific journal has published an article describing how the drug interferes with the virus. While there are no real surprises in what has been revealed, it provides key details on how SARS-CoV-2 can be blocked.
Photocopier
Targeting a virus with a drug is challenging. Viruses make a living by using their host’s proteins to do most of the work of making new viruses. That means that a drug has to target some of the few proteins encoded by the virus without interfering with any of the much more prevalent host cell proteins. In the case of the coronavirus, biologists have identified a number of distinct characteristics of the virus that can be attacked without an obvious risk of causing serious side effects.
Remdesivir, which saw a large clinical trial produce promising results, is a drug designed to attack one of these virus-specific vulnerabilities. The coronavirus genome is encoded using the chemical RNA, unlike the DNA used for our genome. In fact, there is nothing in our cells that forces them to make an RNA copy of an RNA molecule. As a result, the coronavirus genome encodes proteins that make this copy of RNA to RNA, called RNA-dependent RNA polymerase. Remdesivir was designed to look like one of the building blocks of RNA in the hope that it would bind to and inhibit the polymerase of an RNA virus.
That said, this drug was designed with the intention of inhibiting the polymerase of a different virus (Ebola), so it was not guaranteed to work against the coronavirus. And our cells need to make copies of DNA RNA, a process that is similar enough that remdesivir can also interfere with that.
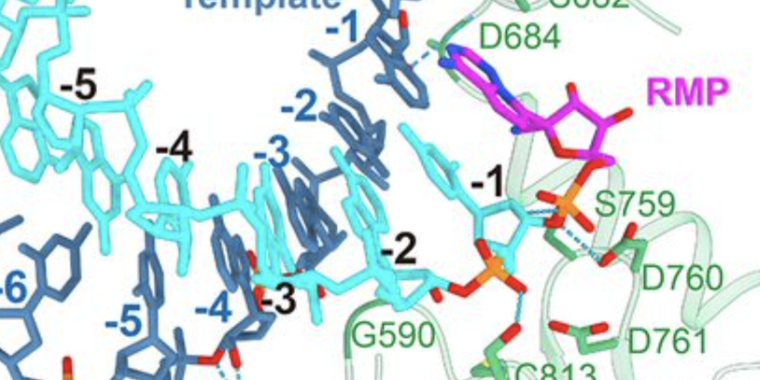
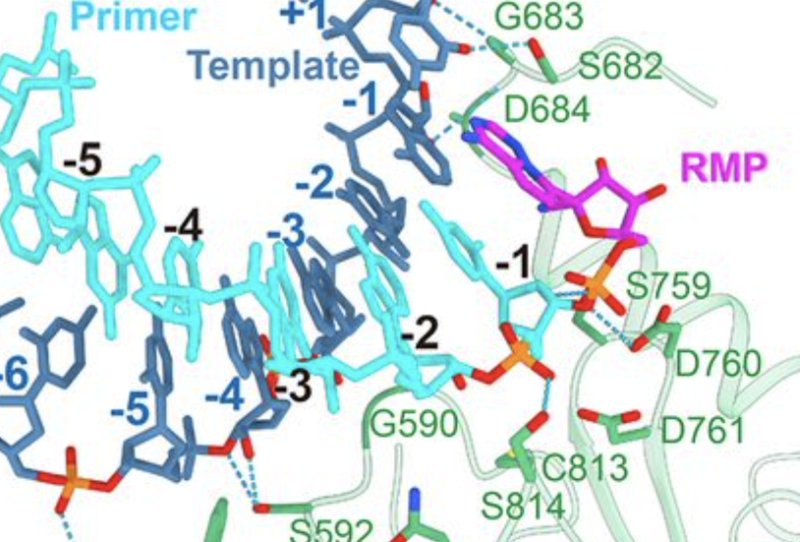
Still, the tests on the cells had been promising enough to conduct human tests. As the tests began, a group of Chinese scientists decided to investigate how remdesivir really works. To do so, they decided to discover how the drug interacted with coronavirus RNA polymerase at the atomic level. And that requires a technique to determine where all the atoms are in the protein and the drug.
A few decades ago, discovering the details of proteins at the atomic level would have required many months of painstaking attempt to get the drug and protein to form clean and orderly crystals. But since then we have developed a combination of hardware and algorithms that now allow us to take what are essentially electron microscope images of individual proteins and combine them with enough precision to discover where all the atoms are. The technique, called cryogenic electron microscopy, has been so revolutionary that it earned the people who developed it a Nobel Prize.
These scientists also benefited from previous work on other coronaviruses, which had identified three different proteins that were critical for copying the virus genome. One of them is the enzyme that actually unites individual units called “bases” to form a new RNA molecule. The other proteins in question simply help you suppress and move along the RNA you make a copy of. The researchers then produced these three proteins, placed an RNA template and a partial copy, and then added remdesivir.
Atoms
Well technically they didn’t add remdesivir. The bases are added to the RNA in a form with three phosphates attached. That form has a very negative charge and will not go through enzymes very easily. Instead, the drug is provided in a minimally loaded form, which can transit through cell membranes. Once inside the cell, the cell’s own enzymes convert it into the charged form, which is then used by the enzyme that copies the RNA. Since they were not working on the cells, the researchers had to do this conversion themselves. It is a bit apart, but it illustrates some of the challenges faced by people who develop medications.
In any case, the imaging technique requires taking thousands of electron micrographs of individual protein-RNA-drug complexes, all randomly oriented. While none of these is by itself sufficient to discover where the atoms are, computer algorithms can combine all of these images and determine which underlying structure is compatible with all of these different images.
In the end, after combining more than 80,000 images, the resolution of the structure they determined had an accuracy of approximately 2.5 angstroms (10-10 meters). For comparison, a carbon atom in these same molecules is about 1.5 angstroms wide. But given the fact that many settings consistent with this resolution don’t make sense (they put two carbon atoms on top of each other or something similar), the image is quite detailed.
One thing they discovered is that the proteins involved have zinc atoms built into their structure. This will not surprise any biochemist, as zinc-containing proteins are common. But there has been a steady stream of complementary treatments for the disease, including some that involve chloroquine derivatives, in which zinc was a key component. We will have to see if that changes now that it is clear that zinc is needed to make copies of the virus (assuming the fact is recorded at all with people prone to promoting marginal therapies).
The structure confirms that remdesivir not only binds to the enzyme and blocks it. Instead, the drug is chemically incorporated into the growing chain of RNA. Once there, however, you don’t have the proper chemistry to add another base later. As a result, the RNA cannot grow anymore. The copy ends and the resulting genome is defective. This is the same type of drug mechanism that was behind some of the first anti-HIV drugs, such as AZT.
Why doesn’t this completely block the virus? Presumably because there is simply much more than the normal equivalent of the RNA building blocks in the cell, and it is difficult to bring the drug to concentrations where it constantly damages all the viral copies being made. An additional limitation is that, once the drug blocks the copy of a molecule, it is essentially deactivated, since it remains chemically linked to that molecule.
The researchers note that there are other similar drugs that bind to the coronavirus RNA polymerase even more effectively. Therefore, there is a possibility that we can get the structure of some of them and determine if there are rules for what kind of chemicals are especially good for binding to the coronavirus enzyme.
Science, 2020. DOI: 10.1126 / science.abc1560 (About DOIs).