
[ad_1]
On Wednesday (9), the British AstraZeneca vaccine trial emergency call card (AstraZeneca), the subject was suspected of having side effects in the clinical trial, leading to safety concerns. The World Health Organization (WHO) responded on Thursday (10), This raises the alarm bell.
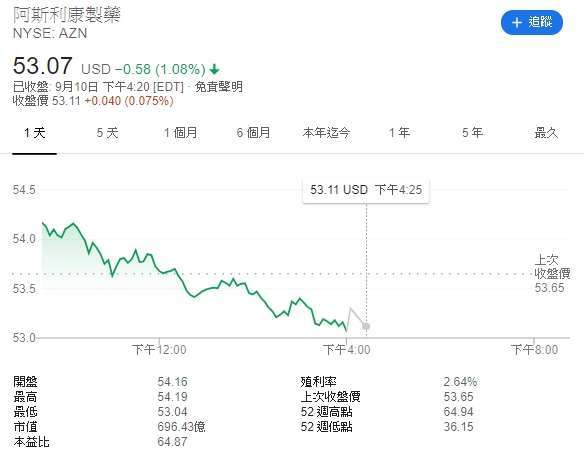
Affected by this news, British pharmaceutical giant AstraZeneca (AZN-US) continued to fall 1.08% to $ 53.07 a share on Thursday, closing in the black for two consecutive trading days.
The global epidemic of new corona pneumonia (COVID-19) continues to spread. Before the deadline, according to real-time statistics from Johns Hopkins University in the United States, the number of confirmed cases worldwide has exceeded 27.8 million and the number of deaths has exceeded 904,000. The United States has accumulated more than 6.36 million cases were diagnosed and the accumulated number of deaths reached 190,000.
A vaccine developed jointly by AstraZeneca and the University of Oxford in the UK suspended the final phase of clinical trials on Wednesday. The main reason is that the patient has a rare unexplained disease (transverse myelitis), which may or may not be related to the vaccine. Irrelevant.
WHO Chief Scientist Soumya Swaminathan responded on Thursday: I think this can be a wake-up call or a lesson to make people aware of the ups and downs of research and clinical processes, and we must be prepared for it.
Vaminatan said there is no need to be overly discouraged by this news. These things happen. This is good clinical practice, because in any clinical trial, safety is the most important thing. If it’s a minor side effect, you need to take action. If there are serious side effects, clinical trials are stopped. This is a normal procedure.

Foreign media cited sources who reported that AstraZeneca (AZN-US) may restart its trials of suspended vaccines next week. AstraZeneca CEO Pascal Soriot insisted that if the trial resumes soon, the company is expected to have the results of the vaccine before the end of the year.
The new crown has triggered great economic and social upheaval around the world. Governments and companies in several countries are eager to end the new corona epidemic and are accelerating vaccine development.
Mike Ryan, WHO Executive Director of Emergency Planning, said: “The discovery and approval of a new corona vaccine is not a race between companies or between countries. It is a race against time, a race against the virus. and a race to save lives.. “
[ad_2]