Complex molecular architectures are generally built by gradually connecting a variety of building blocks. But sometimes, complex structures arise from single-component self-assembly. Writing in NatureDatta et al.one Show how polycathenes, chains made of interlocking rings at the nanometer scale, can be formed by the remarkable self-assembly of a simple molecular building block.
Cathenes are molecules in which two or more molecular rings are entangled like the links in a chain.two; in fact, its name derives from chain, the Latin word for string. The rings are not connected by a covalent bond, but instead form a different type of bond called a mechanical bond, in which the connected rings can move freely around each other. This dynamic property makes them useful as components of artificial molecular machines.3. Many catenans reported so far consist of just two rings. Building molecular chains made of various rings is a major challenge for synthesis, and has been accomplished only in recent years, for small molecular rings (with a radius of about 1 nanometer)4 4.
The construction of larger systems is limited by the efficiency of the cateation step, in which a pre-assembled toroidal precursor forms a ring that interlocks through another toroid; in addition, a large number of covalent bonds must be formed in the pre-assembled structure. Therefore, synthetic routes that involve non-covalent assembly techniques are preferred.5 5. In supramolecular polymerization, for example6 6Simple, self-assembling, molecular building blocks in a single pass through non-covalent interactions to form large-scale structures of varying geometries. Unfortunately, the size gain of assemblies made this way often comes at a price: Chemists have less control over the architecture of the final build, compared to a multi-step covalent strategy.
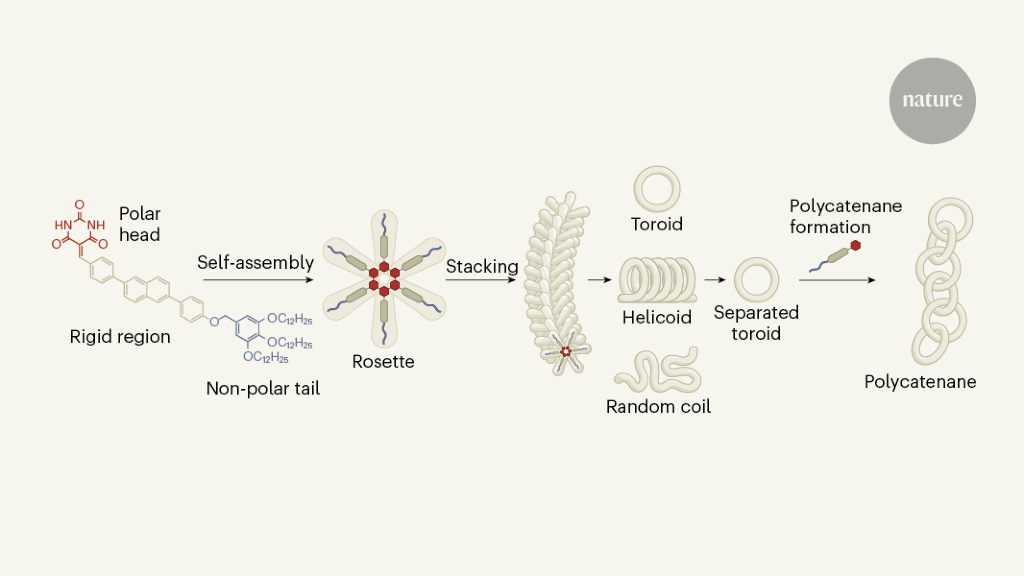
Datta and his co-workers have combined aspects of covalent and non-covalent strategies to form their complex polycane structures. The authors started with a monomer consisting of a polar head and a nonpolar tail, separated by a rigid section consisting of benzene rings (Fig. 1). Six of these monomers can self-assemble in an appropriate solvent to form a star-shaped ‘rosette’. The polar heads form a hexagonal nucleus that is held together by hydrogen bonds, in the same way that DNA helices are held together by hydrogen bonds in nucleotide base pairs, and the rigid sections point outward from the nucleus as arms.
Once formed, the rosettes self-assemble by stacking on top of each other, a process driven by the formation of interactions (known as π–π interactions) between the rigid regions of neighboring rosettes. Because each rosette added to the stack is slightly offset from its predecessor, the resulting assembly grows with an intrinsic curvature that produces various geometries: random, helical, and toroidal coils.7 7. The type of geometry that is formed depends on the cooling rate of the initial monomer solution. Slow cooling (approximately 1 kelvin per minute) favors the formation of helical fibers; faster cooling (about 10K min-one) generates random coils; and sudden cooling adds toroids to the mix.
Datta and his colleagues noted that rapid cooling also produced traces of catenanes consisting of two interlocking toroids. This suggested that the individual toroids could act as secondary sites from which another ring could grow, thus forming the cate dimers. The authors took advantage of this fortuitous process to design a protocol for making large self-assembled polycatennae using a toroid solution as ‘seeds’ for catenification.
The authors quickly chilled a solution of the monomer in a solvent mixture that was chosen to facilitate toroid formation, thereby producing a solution in which approximately half of the monomer molecules were incorporated into the toroids; the remaining monomers were self-assembled into randomly wound linear structures. Because toroids are more heat stable than their linear counterparts, the authors could selectively disassemble the coils into monomers by heating the solution. Subsequent slow cooling promoted the formation of long, helical supramolecular assemblies from the monomers, leaving the toroids intact. Datta et al. He then filtered the mixture to remove long helical structures, thereby producing a solution that contained predominantly toroids.
Finally, the authors produced polycathenes by adding monomers to the toroid solution, which seeded the formation of new catheterized rings, as expected. Nonpolar tails were originally incorporated into monomers to improve monomer solubility, but Datta and colleagues found that they also play a crucial role in the seeding process: unfavorable interactions between the tails and the solvent make it more likely. that rosette self-assembly will start on the surface of existing toroids.
Atomic force microscopy revealed that polycathenes of various sizes are formed in the reactions, and that the toroids have a radius of 12.5 nm. The authors discovered that the addition of monomers in small portions favors the initiation of self-assembly processes that lead to catheting and, therefore, were able to produce linear and branched polytecanes containing up to 22 rings. This is close to the number previously achieved using the covalent assembly (up to 26 rings in linear polycathenes)4 4and further demonstrates the effectiveness of Datta and colleagues’ approach to synthesizing complex, non-covalent structures.
The authors’ protocol also shows that a multi-step approach, taken from the covalent synthesis playbook, can be used to produce large and complex self-assembling architectures in a controllable manner. This is an important step in the development of non-covalent synthesis, and its protocol can be expected to inspire the field to tackle more challenging goals.5 5. It would be interesting to see, for example, if the monomer can be adapted to obtain catheterized rings of various sizes, or if heterocathenes can be made, in which the seed toroid consists of a different type of monomer from which macrocycles with legs are assembled.
It remains to be seen how the mechanical and dynamic properties of self-assembled polycathenes compare with those of their smaller covalent counterparts. A major attraction of covalently assembled cathenes is that, if the relative motion and position of the rings can be controlled, potential applications for molecular machines are opened up. The possibility of achieving the same level of control over large self-assembled structures would bring us a little closer to what nature achieves with cellular machinery.
