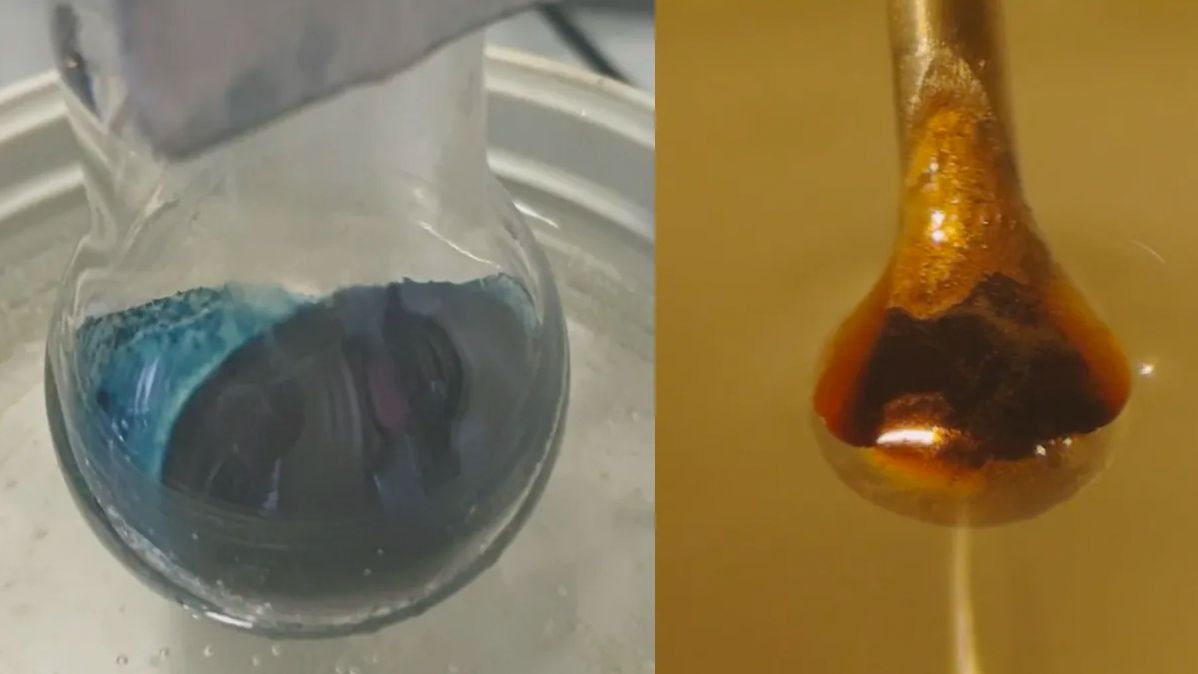
Scientists can finally understand the mysterious transition behind a century-old chemistry experiment. The details of this transformation, in which the addition of electrons to a brilliant blue ammonia solution is transformed into a brilliant metallic bronze, have long eluded scientists.
The new study reveals the subtle details of this change, and shows that this transformation is gradual, rather than sudden. “What we have done successfully is that we have understood quite well how these solutions behave in a wide range of concentrations using a microjet technique,” said study co-author Ryan McMullen, a doctoral student in chemistry at the University of Southern California. California. This technique, which involves firing thin streams of the solution through a vacuum, has never been used in the shiny liquid before.
And the discovery could open up new kinds of reactions in organic chemistry in the future, McMullen told Live Science.
Related: 8 chemical elements you’ve never heard of
Rails They are a diverse group. Something like lithiumThey are light enough to float, while others, such as lead or osmium, are extremely dense. Some require incredibly high temperatures to melt, while others melt easily (MercuryFor example, it melts at minus 38.3 degrees Celsius, or minus 37.9 degrees Fahrenheit.) Ultimately, what metals have in common is their ability to conduct electricity at absolute zero, the point at which the molecular movement of heat essentially stops.
But how are some nonmetals transformed into metals? In a new study, researchers answered that question by adding metals to liquid ammonia.
First, the researchers condensed ammonia, which is a gas at room temperature, into a liquid, cooling it to 27.4 F (minus 33 C) negative. Then they added any sodium, lithium or potassium, which are all alkali metals. (Rather famous, these metals react explosively when immersed in water). The experiments were carried out in collaboration with scientists from the Czech Academy of Sciences and the Fritz-Haber Institute of the Max Planck Society in Berlin, as well as researchers in Japan and France.
Related: The 10 best explosions in history
The result was an expected reaction: Liquid ammonia extracted electrons from the metal. Those electrons were trapped between the ammonia molecules, creating the so-called solvated electrons that the researchers hoped to study. At low concentrations, the result was a blue non-metallic liquid. However, as the solvated or trapped electrons accumulated, the solution turned into bright bronze.

The next challenge was to investigate how the solvated electrons behaved at different concentrations. This involved firing a microjet of the solution, about the width of a human hair, through a synchrotron beam X-rays, which are high-energy X-ray beams. X-rays excited the solvated electrons, causing them to jump out of their liquid cage of ammonia molecules. The researchers were then able to measure the amount of energy needed to release the solvated electrons.
The researchers found that the higher the concentration of solvated electrons, the more the pattern of energy release matches what is seen in a metal. Here’s what that means: If you graph the amount of energy required to release electrons from your liquid ammonia cage, metals generally have what is called a “Fermi edge,” a very abrupt transition, McMullen said. At lower concentrations of solvated electrons, this energy release graph looks more like a rounded hill. Only at higher electron concentrations did this Fermi edge emerge. The border reflects how much energy the electrons have at a given temperature, McMullen added.
“When you increase the concentration to the metallic range, then you see, this wonderful pattern arises that is very, very characteristic of a metal,” said McMullen.
The results were interesting because they showed that the metal-like liquid created by combining alkali metals and ammonia is actually a metal on a fundamental physical level, he said.
“It’s a genuine metal, it’s not something that looks like one,” said McMullen.
The lower concentration solvated electrons are used in a type of reaction called the Birch reaction, which adds electrons to molecular structures called aromatic rings. This type of reaction was used in the manufacture of the first oral contraceptive pills in the 1950s, McMullen said. By understanding how solvated electrons work at high concentrations, researchers can potentially find new types of chemical reactions, he said. For example, they could excite the solvated electrons with light beams to behave in new ways.
“If you tickle the electrons a little bit to make them more energetically excited, you can start to see some crazy reactions that would otherwise never happen,” McMullen said.
The researchers reported their findings June 5 in the journal. Science.
Originally published in Live Science.