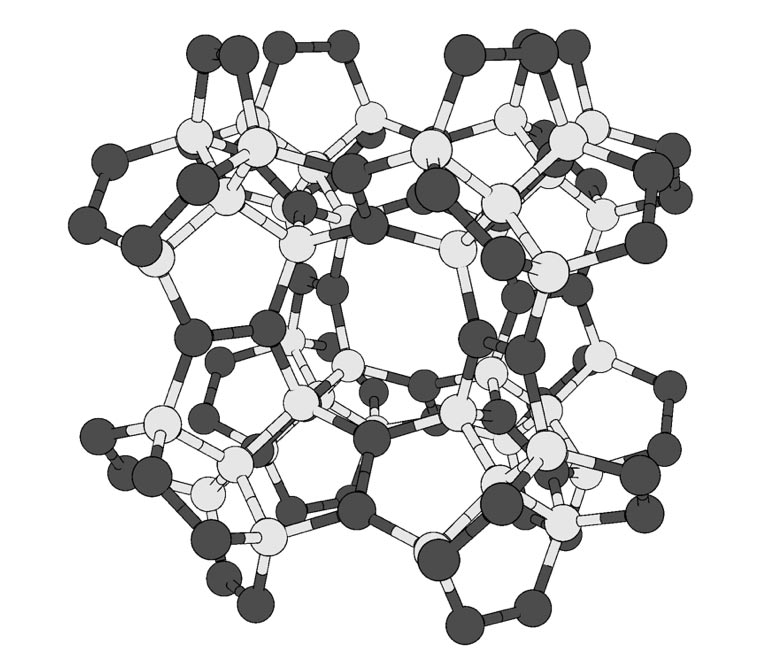

Geometric pentadiamond structure. White and black balls indicate C atoms with four and three adjacent C atoms, respectively. Credit: Tsukuba University
Scientists at Tsukuba University use computer calculations to propose a new way to rearrange carbon atoms in a diamond to make it even more difficult, which can be useful in industrial applications that rely on synthetic cut diamonds.
Researchers at Tsukuba University used computer calculations to design a new carbon-based material even harder than diamond. Called “pentadiamond” by its creators, this structure can be useful to replace today’s synthetic diamonds in difficult cutting manufacturing tasks.
Diamonds, which are made entirely of carbon atoms arranged in a dense network, are famous for their hardness unmatched among known materials. However, carbon can form many other stable configurations, called allotropes. These include the familiar graphite in the pencil mine, as well as nanomaterials like carbon nanotubes. The mechanical properties, including the hardness, of an allotrope depend mainly on the way in which its atoms are joined together. In conventional diamonds, each carbon atom forms a covalent bond with four neighbors. Chemists call carbon atoms that have sp3 hybridization. In nanotubes and some other materials, each carbon forms three bonds, called sptwo hybridization.

Pentagons merged as a constituency of pentadiamond. Credit: Tsukuba University
Now, researchers at Tsukuba University have explored what would happen if carbon atoms were arranged in a more complex structure with a mixture of sp3 and sptwo hybridization.
“Allotropes of carbon with both sptwo and sp3 hybridized atoms have greater morphological diversity due to the large number of combinations and arrangements in the networks, “says first author Yasumaru Fujii.
To calculate the most stable atomic configuration, as well as to estimate its hardness, the team relied on a computational method called functional density theory (DFT). DFT has been used successfully in solid state chemistry and physics to predict the structure and properties of materials. Keeping track of the quantum states of all the electrons in a sample, and especially their interactions, is often an insoluble task. Instead, DFT uses an approximation that focuses on the final density of electrons in the space that orbits the atoms.
This simplifies the calculation to make it suitable for computers, while providing highly accurate results. The scientists found that Young’s modulus, a measure of hardness, for pentadiamond was nearly 1,700 GPa, compared to about 1,200 GPa for conventional diamond.
“Not only is pentadiamond harder than conventional diamond, its density is much lower, equal to that of graphite,” explains co-author Professor Mina Maruyama. “This work shows the power of designing materials ab initio. In addition to industrial cutting and drilling uses, pentadiamonds could be used in place of the diamond anvil cells that are currently used in scientific research to recreate extreme pressure within planets, “said senior co-author Professor Susumu. Okada.
Reference: “Pentadiamond: a hard carbon allotrope from a pentagonal network of sptwo and sp3 C Atoms “by Yasumaru Fujii, Mina Maruyama, Nguyen Thanh Cuong and Susumu Okada, June 30, 2020, Physical Review Letters.
DOI: 10.1103 / PhysRevLett.125.016001