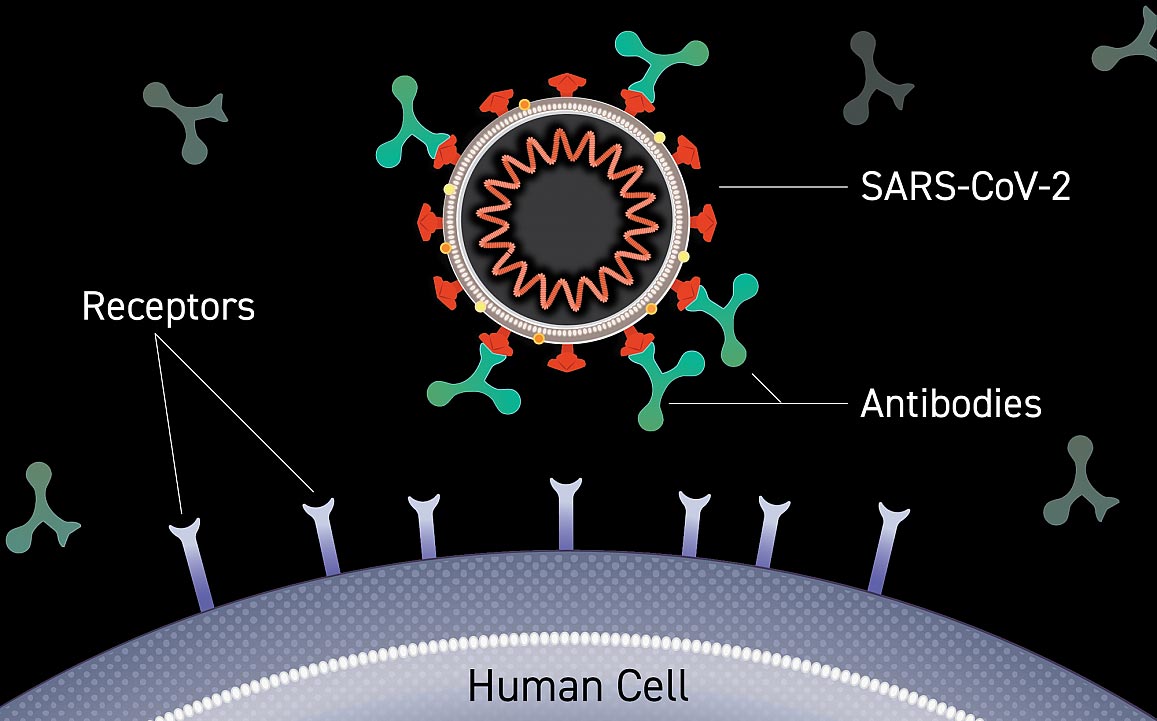

Imaging of an antibody bound to the surface of a virus blocks entry into a human cell. Credit: Lisa Donohue, CoVPN
Two Phase 3, randomized, placebo-controlled, double-blind clinical trials testing whether experimental monoclonal antibody (mAbs) infection can be prevented by SARS-CoV-2 coronavirus is now registering healthy adults at clinical trial locations in the United States. Many of the test sites and research researchers are part of the COVID-19 Prevention Network (CoVPN), recently established by the National Institute of Allergy and Infectious Diseases (NIAID), one of the National Institutes of Health. SARS-CoV-2 is the virus that will cause coronavirus disease 2019 (COVID-19). The tears register adults who are at risk of infection due to close contact at work or at home to individuals with SARS-CoV-2 infection.
“The COVID-19 Prevention Network is designed to perform large-scale trials rapidly and efficiently,” said NIAID Director Anthony S. Fauci, MD. “This network will allow us to test the safety and effectiveness of monoclonal antibodies and other preventive measures to help identify how best to reduce the level of SARS-CoV-2 infection and ultimately end the COVID-19 pandemic. ”
Monoclonal antibodies are laboratory-made versions of proteins that are naturally produced by the immune system in response to invasive viruses or other pathogens. Neutralizing antibodies, or naturally as monoclonal, can bind directly to parts of viruses that they use to hook up and enter cells, preventing them from starting the infection cycle. Monoclonal antibodies can provide short-term protection from SARS-CoV-2 and could serve as key components of the COVID-19 pandemic response until vaccines are available.
One trial is being conducted jointly by NIAID and trial sponsor Regeneron Pharmaceuticals of Tarrytown, New York. It will evaluate Regeneron’s research double mAb combination, REGN-COV-2, which is designed to bind to two points on the SARS-CoV-2 spike protein and prevent it from entering healthy cells. The trial will enroll about 2,000 asymptomatic adults who are domestic contacts of individuals with SARS-CoV-2 infection. Participants must have been in close contact (typically due to living at the same address) with the infected person in a 96-hour window prior administration of REGN-CoV-2 as a placebo. In addition to assessing safety, the trial will attempt to determine whether REGN-COV-2 infections may occur as disease symptoms in those who are already infected. The efficacy assessment will be a period of one month after administration of REGN-COV-2 as a placebo. All subjects will be monitored for seven months for safety after completion of the efficacy assessment period.
Additional details about this trial are available at clinicaltrials.gov using identifier NCT04452318. Interested parties can also visit the CoVPN website for details. Physicians or potential participants can also contact the Clinical Trials Admin Administrator at 844-734-6643 or [email protected] for enrollment information.
A second trial, sponsored by Eli Lilly and Company of Indianapolis, Indiana, and conducted in collaboration with NIAID, will evaluate LY-CoV555, a mAb isolated from a recovered patient COVID-19 by scientists at AbCellera (Vancouver, British Columbia, Canada) and the NIAID Vaccine Research Center, and developed by Eli Lilly and Company. This trial will assess whether LY-CoV555 SARS-CoV-2 infection can occur among people at high risk of exposure due to living or working in knowledgeable nursing or assisted living facilities. Within one week of identifying a case of SARS-CoV-2 infection at a facility, researchers will enroll study volunteers and evaluate the effectiveness and safety of prevention of LY-CoV555, compared to placebo, over a period of 8 weeks. The test will also assess effectiveness in preventing symptoms of a given severity in those who are already infected. Participants will continue to be followed for safety for an additional 16 weeks. Up to 2,400 participants will be randomized to receive intravenous infusion of LY-CoV555 as a placebo.
Additional information about this trial can be obtained from clinicaltrials.gov using identifier NCT04497987. Clinical investigators, hospitals or clinical sites interested in participating in one of Lilly’s clinical trials for a potential COVID-19 treatment should call 1-877-CT-LILLY (1-877-285-4559) by e-mail [email protected].
NIAID conducts and supports research – at NIH, throughout the United States, and worldwide – to study the causes of infectious and immune-mediated diseases, and to develop better resources for the prevention, diagnosis, and treatment of these diseases.
About the National Institutes of Health (NIH), the nation’s medical research bureau, comprises 27 institutions and centers and is part of the U.S. Department of Health and Human Services. NIH is the primary federal agency that conducts and supports basic, clinical, and translational medical research, and investigates the causes, treatments, and cures for both common and rare diseases.