FDA Commissioner Dr. Steven Hahn told the Financial Times that his agency may consider emergency use authorization for the Covid-19 vaccine before authorization is completed at a late stage clinical trial, but his concern is that the FDA Can grow rapidly. Show strong enough evidence that it will protect people.
Vaccine approval
Otherwise, vaccines have to go through a full clinical trial process and FDA approval process, which can take months or years.
When the process of making a vaccine has been speeded up, bad results have come.
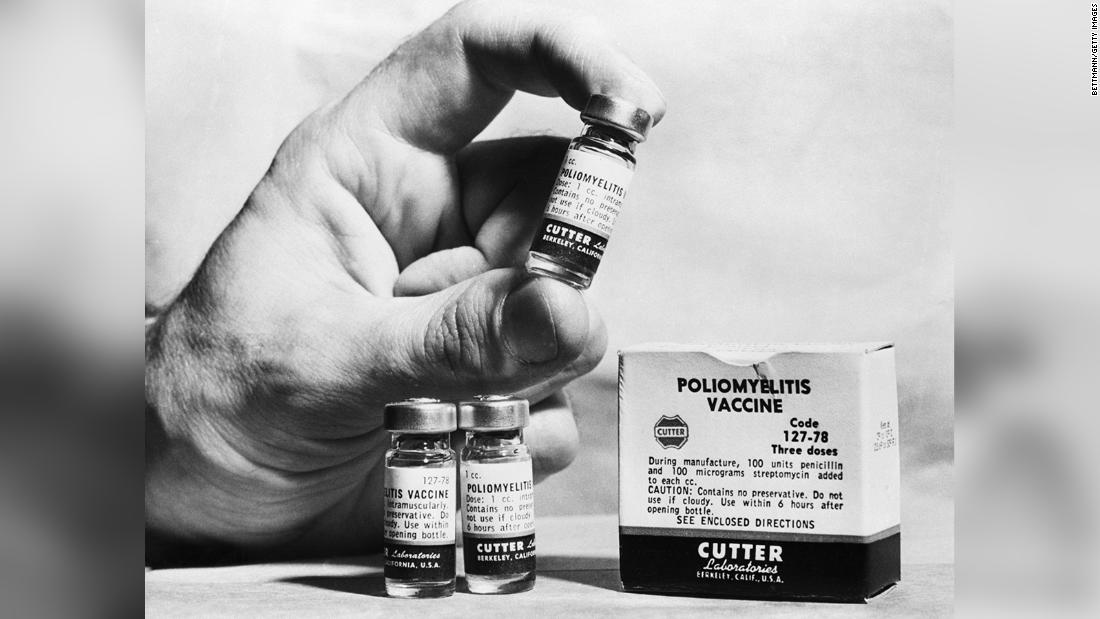
Cutter phenomenon
On April 12, 1955, the government announced the first vaccine to protect children against polio. Within days, laboratories had created thousands of vaccines. The batch, created by a company called Cutter Labs, contained a live polio virus and caused an outbreak.
More than 200,000 children received the polio vaccine, but within days the government dropped the program.
“Forty thousand children had polio. Some had low levels, some a hundred were paralyzed, and about 10 people died,” said Dr. Dr. Howard Markel, Pediatrician, Distinguished Professor and Director of the Center for History Medicine. University of Michigan. The government suspended the vaccination program until it could determine what went wrong.
The monkey’s trouble
However, increased monitoring failed to find another problem with the polio vaccine.
“The way they spread the virus was on monkey tissue. These rhesus macaques were brought from India, thousands of them,” said medical anthropologist S. Lochlan Jain said. Jain, who teaches the history of the vaccine course at Stanford and is working on a publication, added, “They were gang cages and in that situation, many people who did not die on the journey fell ill, and the virus spread quickly.” About the event. Scientists mistakenly thought that the formaldehyde they used would kill the virus. “Millions of Americans were being evacuated,” Jain said.
The CDC says no current vaccine contains the SV40 virus, and there is no evidence that the contamination could harm anyone.
Which was never an epidemic
In 1976, scientists predicted a new strain of influenza called swine flu. More than 40 years later, some historians call it the “flu pandemic that never happened.”
“President Ford was basically told by his advisers that we have the epidemic flu, called swine flu, which can be as bad as the Spanish flu,” said Michael Kinch, a professor of radiation oncology at the School of Medicine at Washington University. St. Louis. His latest book, Between Hope and Fear, explores the history of vaccines.
“Ford was rushing to get a vaccine urgently. When you had a new stress situation, they had to do it on the fly,” Kinch said.
Ford decided to make immunity mandatory.
According to the CDC, the government launched the program in about seven months and 40 million people were vaccinated against swine flu. That vaccination campaign was later linked to cases of a neurological disorder called Guillain-Barr સિ syndrome, which can develop after infection or, rarely, after vaccination with a live vaccine.
“It was a fiasco,” Merkel said. “The good news is that there has never been an outbreak of swine flu. So we were safe, but it shows you what can happen.”
U.S. Growing distrust in
It took a lot of events for people to start an incredible vaccine. Even after thousands of children fell ill from the first polio vaccine in 1955, when the program resumed, parents made sure their children were vaccinated. They have vivid memories of an epidemic that paralyzes 13,000 to 20,000 children each year. Some were so paralyzed that they could not even breathe easily and relied on machines called iron lungs to help them breathe.
“Parents were forcing their children to go to the head of the line to get the polio vaccine, because they had been seeing epidemics every summer for years and seeing children in iron lungs and they were panicking.”
Merkel said people’s attitudes began to change between the 1955 and 1976 swine flu vaccination projects.
“You’ve got civil rights, when you see people beating the hell out of people on TV. You’ve got the Vietnam War where people seem to be offended by the killings. You’ve got Watergate when the president is literally lying.” “This led to real distrust of officials and the federal government, and it spread to doctors and scientists,” Merkel said. And, over time, that is likely to change. “
A ‘very stupid’ move
Merkel said people’s distrust of the system suggests that the FDA will move forward with the process before the end-stage clinical trial is completed. “Extremely stupid.”
“This is one of the most ridiculous things I’ve ever heard this administration say,” Merkel said. “All it takes is a bad side effect to basically bootch a vaccine program that we desperately need against this virus. It’s a prescription for disaster.”
FDA Commissioner Hahn said the vaccine decision would be based on data, not politics, but shared Kinch Merkel’s concerns.
This could lead to significant damage, Kinch said. Kinch, who himself is a patient in a vaccine test, said the clinical trial process needs to be followed to the end. The EU too early for the vaccine could produce a “nightmare” for a few reasons.
One, the vaccine may not be safe. Two, if it is not safe, people will lose faith in vaccines. Three, if a vaccine does not provide complete protection, people will have a false sense of safety and the risk will increase. Four, if the substandard vaccine gets EUA, a better vaccine can never be approved, as people will be reluctant to register for the test and risk taking a placebo instead of the vaccine.
“People will die unnecessarily if we take chances with this,” Kinch said. “We have to get this right.”
.