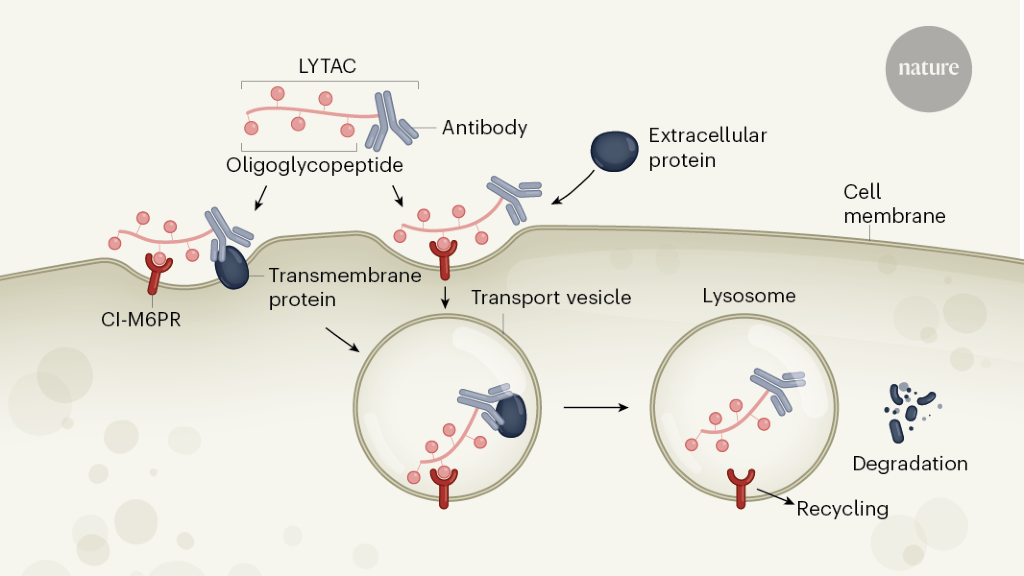
Most drugs work by binding to a specific site on a target protein to block or modulate protein function. However, the activity of many proteins cannot be altered in this way. Instead, an emerging class of drugs brings proteins closer to other molecules, which then alter protein function in unconventional ways.one–3. One of these approaches uses drug molecules called protein degraders, which promote protein labeling with ubiquitin, another small protein. The labeled proteins are broken down into small peptide molecules by the cell’s proteasome machinery. But because the ubiquitin-mediated degradation pathway occurs within the cell, the protein breakers developed thus far primarily target intracellular targets. Writing in NatureBanik et al.4 4 they now report a different mechanism that opens membrane-bound and extracellular proteins for targeted degradation.
The authors report protein breakers called lysosome-directed chimeras (LYTAC), which are bifunctional (they have two binding regions; Fig. 1). One end carries an oligoglycopeptide group that binds to a transmembrane receptor (the cation-independent mannose-6-phosphate receptor; CI-M6PR) on the cell surface. The other end carries an antibody or a small molecule that binds to the protein destined for destruction. These two regions are linked by a chemical linker.
The formation of a CI-M6PR-LYTAC-target trimer complex in the plasma membrane drives destruction of the complex by protease enzymes in membrane-bound organelles called lysosomes. LYTACs are conceptually related, but complementary, to chimeras targeting proteolysis5 5 (PROTAC): Another bifunctional class of protein breakers that targets primarily intracellular proteins by recruiting them for E3 ligases (the enzymes that label proteins with ubiquitin).
Banik et al. He started by making LYTACs of different sizes and binding composition, and which used a small molecule called biotin as a protein binding component: Biotin binds with exceptionally high affinity to avidin proteins. The authors noted that these LYTACs rapidly transported an extracellular fluorescent avidin protein to intracellular lysosomes in a manner that required commitment to CI-M6PR. When the authors replaced biotin with an antibody that recognizes apolipoprotein E4 (a protein implicated in neurodegenerative diseases), this protein was also internalized and degraded by lysosomes. LYTACs can therefore reuse antibodies from their normal immune function to target extracellular proteins for lysosomal degradation.
Then Banik et al. investigated whether LYTACs could induce degradation of membrane proteins that are targets for drug discovery. In several cancer cell lines, LYTACs induced internalization and lysosomal degradation of the epidermal growth factor receptor (EGFR), a membrane protein that drives cell proliferation by activating a signaling pathway. Depletion of EGFR levels by LYTAC in cancer cell lines reduced activation of the downstream EGFR signal, compared to the amount observed when EGFRs were blocked by antibodies alone. This result confirms a previous report5 5 advantage of using target degradation in therapeutic applications, rather than target blocking.
Similar results were seen with LYTAC for other single-pass transmembrane proteins (proteins that span the cell membrane only once), including programmed death ligand 1 (PD-L1), which helps cancer cells evade the immune system. The next step will be to establish whether LYTACs can also induce the degradation of multi-step proteins that cross the membrane several times, such as the ubiquitous G protein-coupled receptors and proteins that transport materials through the membranes (ion channels and carrier of solutes). proteins, for example). If so, it will be interesting to compare the performance of LYTAC, which would bind to the extracellular domains of such proteins, with that of PROTAC, which can bind to the intracellular domains of these proteins (as recently demonstrated6 6 for solute-bearing proteins).
As with any new form of medication, there is room for improvement. For example, the first LYTACs targeting PD-L1 by Banik and colleagues produced only partial degradation of the protein, which the authors attributed to the low expression of CI-M6PR in the cell lines used. When the authors developed a second type of LYTAC that incorporated a more potent PD-L1 antibody, the degradation increased, albeit in cells that expressed higher levels of CI-M6PR than the original cell lines. This shows that the low abundance of the LYTAC-sequestered lysosome transfer receptor (in this case, CI-M6PR) can reduce the effectiveness of these degraders. Similarly, the loss of core components of E3 ligases is a common mechanism by which cells become resistant to PROTAC.7 7. Lysosome receptors other than CI-M6PR could be used by LYTACs as alternatives, should resistance arise. Degradators targeting cell type specific receptors may also have improved safety profiles compared to conventional small molecule therapeutics, which are not always cell type selective.
What differentiates PROTAC and LYTAC from conventional drugs is their mode of action. For example, after a PROTAC has caused destruction of a target protein, PROTAC is released and can induce additional cycles of ubiquitin labeling and degradation, thus acting as a catalyst at low concentrations.one,5 5. Mechanistic studies are now warranted to determine if LYTACs also work catalytically.
Another aspect of both PROTAC and LYTAC mode of action is that they unite two proteins to form a trimeric complex. A general characteristic of such processes is the hook effect, by which the formation of trimers, and therefore the associated biological activity, decreases at high drug concentrations. This is because dimer complexes generally form preferentially at high drug concentrations, an undesirable effect that can be alleviated by ensuring that all three components interact in such a way that trimer formation is more favorable than dimer formationone.
Kinetics is also important for protein breakers. For example, long-lasting, stable trimeric complexes involving PROTAC accelerate target degradation, improving drug potency and selectivity.8. It will be crucial to understand how the complexes formed by LYTAC can be optimized to improve degradation activity.
PROTAC and LYTAC are larger molecules than conventional drugs. As a result of their size, PROTACs often do not penetrate well through biological membranes, which can make them less potent than the biologically active groups they contain. Size should be less of an issue for LYTACs because they do not need to cross the cell membrane, although they would still have to cross biological barriers to combat diseases of the central nervous system. The development of lysosomal degraders that are smaller and less polar than LYTACs, and therefore better able to pass through membranes, will be eagerly anticipated. Small “glue” molecules that bind to E3 ligases can already do the same job as PROTAC9.
Targeted protein degradation is a promising therapeutic strategy, and the first PROTACs are currently in clinical trials.10. LYTACs will need to catch up, but they have earned their place as a prepared tool to expand the range of proteins that can be degraded. Their development as therapies will require an understanding of their behavior in the human body: their pharmacokinetics, toxicity, and how they are metabolized, distributed, and excreted, for example. Optimizing the biological behavior of molecules that incorporate large groups, such as antibodies and oligoglycopeptides, during drug discovery can be challenging, but this problem can be overcome by further engineering the structures of these groups.eleven. Banik and colleagues’ new degradation approach, therefore, ensures a practical cover approach.
Scientists working on drug discovery will look forward to the development of LYTAC and the emergence of other methods for drug-induced protein degradation.12. Are there no proteins out of the reach of degraders?
