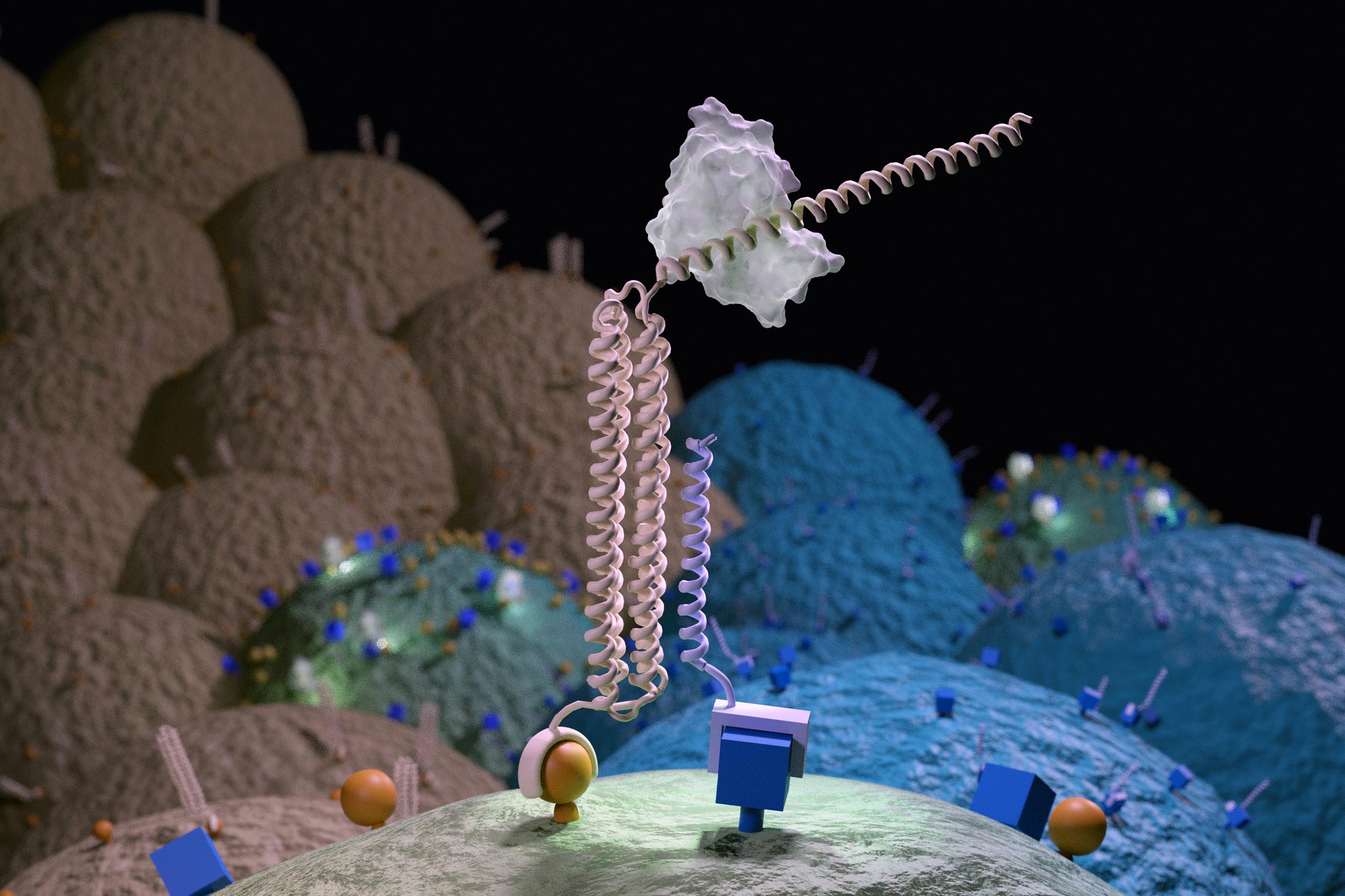

An image of an artist from a Co-LOCKR nano-device coming together on the surface of a cell that has the correct combination of surface markers. Credit: UW Medicine Institute for Protein Design
A laboratory breakthrough in cell targeting could improve the safety of CAR T cells with cancer.
Scientists have shown a new way to precisely target cells by distinguishing them from adjacent cells that look quite similar.
Even cells that become cancerous can differ from their healthy neighbors in only a few subtle ways. A central challenge in the treatment of cancer and many other diseases is the ability to spot the right cells while saving everyone else.
In a paper published today (August 20, 2020) in Science, a team of researchers at the University of Washington The School of Medicine and the Fred Hutchinson Cancer Research Center in Seattle describe the design of new nanoscale devices made from synthetic proteins. These target a therapeutic agent only to cells with specific, predetermined combinations of cell surface markers.
Remarkably, these ‘molecular computers’ all operate on their own and can search the cells they were programmed to find.
“We were trying to solve a major problem in medicine, which is how to target specific cells in a complex environment,” said Marc Lajoie, lead author of the study and recent postdoctoral scientist at the UW Medicine Institute for Protein Design. “Unfortunately, most cells lack one surface mark that is unique to them alone. So, to improve cell targeting, we created a way to target almost every biological function to each cell by going to combinations of cell surface markers. ”
The tool they created is called Co-LOCKR, as colocalization-dependent Latching Orthogonal Cage / Key pRoteins. It is made up of multiple synthetic proteins that, when separated, do nothing. But when the pieces meet on the surface of a straight cell, they change shape, activating a kind of molecular beacon.
The presence of these beacons on a cell surface can lead to a predetermined biological activity – such as cell killing – to a specific, target cell.
The researchers demonstrated that Co-LOCKR can focus the cell-killing activity of CAR T cells. In the lab, they mixed Co-LOCKR proteins, CAR T cells, and a pool of potential target cells. Some of these had only one marker, others had two or three. Only the cells with the predetermined marker combination were killed by the T cells. If a cell also had a predetermined “healthy marker”, then that cell was spared.
“T cells are extremely efficient killers, so the fact that we can limit their activity on cells with the wrong combination of antigens, and still eliminate cells with the right combination changes game,” said Alexander Salter, another lead author of ‘ the study and an MD / Ph.D. student in the program of medical scientists at the UW School of Medicine. He trains in Stanley Riddell’s lab at the Fred Hutchinson Cancer Research Center.
This cell targeting strategy is completely dependent on proteins. This approach distinguishes it from most other methods that rely on engineered cells and operate on slower time scales.
“We believe Co-LOCKR will be useful in many areas where precise cell targeting is needed, including immunotherapy and gene therapy,” said David Baker, Professor of Biochemistry at the UW School of Medicine and Director of the Institute for Protein Design .
###
Reference: August 20, 2020, Science.
DOI: 10.1126 / science.aba6527
This research was conducted at the Institute of Protein Design, the Immunotherapy Integrated Research Center at the Fred Hutchinson Cancer Research Center, and the UW Department of Bioengineering.
The co-lead authors of this work are Marc J. Lajoie (supported by a Washington Research Foundation Innovation Postdoctoral Fellowship and a Cancer Research Institute Irvington Fellowship of the Cancer Research Institute), Scott E. Boyken (supported by the Burroughs Wellcome Fund Career Award at the Scientific Interface), and Alexander I. Salter (supported by the Hearst Foundation and Fred Hutchinson Cancer Research Center Interdisciplinary Training Grant in Cancer Research).
This work was also supported by the National Institutes of Health, National Science Foundation, the Defense Threat Reduction Agency, Nordstrom Barrier Institute for Protein Design Directors Fund, Hearst Foundation, Washington Research Foundation and Translational Research Fund, Howard Hughes Medical Institute, Open Philanthropy Project, and The Audacious Project organized by TED.
Several authors are inventors of patents related to this work. Some hold equity in Lyell Immunopharma. Some authors are now employees as advisors to Lyell Immunopharma.