The Tennessee-based pharmaceutical company announced Wednesday that it is calling two drugs “voluntarily” – one that treats erectile dysfunction, the other depression – after a “product mix”, when the drugs were “inadvertently packaged together,” a third. -Parts are bottled at the facility.
The company’s U.S. In an ad posted on the Food and Drug Administration’s website, the company said Avank, which is headquartered in Pulaski, Ten, recalls several sildenafil 100 mg tablets and several Trezodon 100 mg tablets.
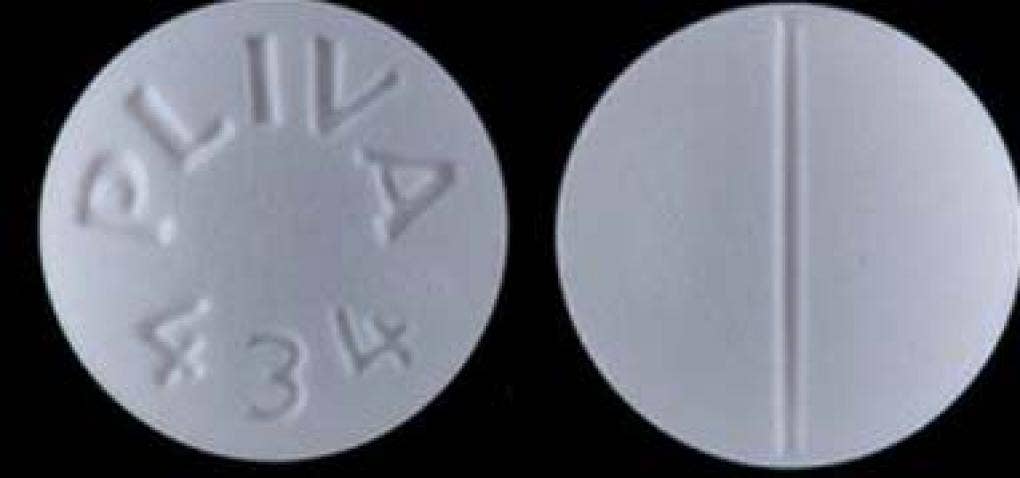
Sildenafil Viagra contains the active ingredient, which is a PDE-5 inhibitor, and is used to treat erectile dysfunction and is packaged in 100-count bottles, the company said.
Electrical dysfunction drugs can help reduce early death from colon cancer, find studies.

Returning drugs “inadvertently packed together.”
(FDA)
According to Avkare, inadvertent use of sildenafil can pose serious health risks to consumers with underlying medical problems. Sildenafil, for example, may interact with nitrates found in some prescription drugs, such as nitroglycerin, which lowers blood pressure to dangerous levels. Consumers with diabetes, high blood pressure or heart disease often take nitrates.
Trezodone hydrochloride is indicated for the treatment of major depressive disorder and is packaged in 1000-count bottles, the company said.

The affected products were distributed across the country.
(FDA)
Unnecessary intake of trazodone can lead to adverse health consequences such as blurred vision, dizziness, constipation and blurred vision – according to Avanke, the risk is higher in elderly patients due to excessive risk and impairment of driving. .
Viral TickTalk Challenge Highlights Stress, Depression, Anxiety Testing
Lots of Sildenafil 100 mg tablets affected – Lot 36884 with expiration date of 03/2022 – and Trazodon hydrochloride 100 mg tablets – Lot 36783 with expiration date of 06/2022 – “Distributed to our distributors and wholesalers, and then distributed nationwide Done, ”the company said.

Avaker said he has notified distributors and customers of the error.
(FDA)
Avker added that it has “notified its distributors and customers and is making arrangements for the return of many of the listed products back.”
At the time the press list was published on Wednesday, Avkar had not received any reports of adverse events related to the recall.

As of Wednesday, no adverse reactions regarding the recall had been reported.
(FDA)
It advises any client who believes they have experienced any problems related to taking these medications to contact their physician or healthcare provider. Adverse reactions or quality problems experienced with the use of this product can be reported to the FDA’s Medwatch Anti-Event Reporting Program either online, by regular mail or by fax.
Click here to get the Fox News app