How can neurons that degenerate in the substantia nigra region of the midbrain be replaced in Parkinson’s disease? An exciting possibility would be to convert non-neuronal cells called astrocytes, which are abundant in the brain, into neurons. Writing in NatureQian et al.one report a simple strategy that takes advantage of this possibility and can improve neurological deficits in a mouse model of Parkinson’s diseaseone. His work, along with a parallel approach recently outlined by Zhou et al. in CelltwoIt holds great promise for our ability to use cell conversion strategies to treat neurodegenerative diseases.
Cell types such as skin cells or astrocytes can be converted through the forced expression of transcription factors, microRNAs or small molecules. – to other types of cells in vitro3–8, including the neurons that make the neurotransmitter molecule dopamine5 5,8; These neurons are lost in Parkinson’s disease. This approach has also been used to convert astrocytes from the mouse brain into neurons. Live6 6–8. For example, astrocytes in the striatum of the brain have become ‘induced dopamine-releasing’ (iDA) neurons that can partially correct motor defects in a mouse model of Parkinson’s disease8. However, iDA neurons generated with this approach did not form the distant neural connections found in a healthy brain, nor did they completely restore motor behavior. Qian et al. and Zhou et al. They have used an alternative strategy to efficiently reprogram astrocytes into neurons: depletion of an RNA-binding protein called PTB that is expressed in astrocytes and inhibits neuronal differentiation.
Qian et al. they started their experiments in vitro, using astrocytes isolated from the cortex and midbrain of the brains of mice, and from the human cortex. The authors used an RNA molecule called small hairpin RNA to promote the degradation of the transcribed messenger RNA of the gene encoding PTB, Ptbp1. This caused the conversion of all three types of astrocytes to neurons. Zhou et al. achieved the same effect using the CRISPR-CasRx genome editing technique to exhaust Ptbp1 MRNA in astrocytes isolated from the mouse cortex.
Then the two teams sold out PTB Live in the brain of the adult mouse. Qian and his colleagues used genetically modified mice so that the small astrocytes could be attacked by the small hairpin RNA Ptbp1, which was carried to the brain in a viral construct. On the contrary, Zhou et al. Astrocytes infected in wild-type mice with a virus carrying the CRISPR-CasRx machinery. Both strategies led to the conversion of astrocytes targeting neuronal cell types.
The groups then depleted PTB in a mouse model of Parkinson’s disease. In these animals, as in people with the disorder, dopamine-releasing neurons are depleted in the substantia nigra, and dopamine levels are abnormally low in the striatum (the area to which these neurons are projected). resulting in deficits in motor behavior. Qian et al. PTB depleted in astrocytes in the substantia nigra of these animals; Zhou et al. In the striated body. Both approaches yielded the same result: conversion of some of the infected astrocytes to neurons that resembled those lost in Parkinson’s disease, and restoration of motor behavior.
Both groups demonstrated that PTB depletion causes astrocytes to become neural cell types, largely appropriate to the region of the brain in which they reside. How is this specificity transmitted? Qian et al. found that astrocytes in the midbrain express low levels of transcription factors Lmx1a and Foxa2; these are expressed in the progenitors of dopamine-releasing neurons during midbrain development and are necessary for the maturation of these progenitors in neurons9. PTB depletion further increased the expression of these factors in midbrain astrocytes. In contrast, in cortical astrocytes, treatment led to higher levels of transcription factors associated with cortical neurons, such as Ctip2 and Cux. Furthermore, reprogramming of astrocytes in the substantia nigra, or in the neighboring ventral tegmental area, produced different subtypes of the iDA neuron that express subtype-specific transcription factors and proteins: Sox6 and Aldh1a1 in the substantia nigra, Otx2 in the ventral tegmental area. .
Qian and colleagues’ results indicate that brain region-specific transcription factors contribute to the conversion of astrocytes to iDA. However, such a mechanism cannot explain why Zhou et al. they were able to convert striatal astrocytes to iDA neurons, since striatal astrocytes express a different set of region-specific transcription factors. What could be the mechanism leading to iDA conversion in the striatum?
Zhou and colleagues show a nearly three-fold increase in iDA conversion efficiency in the Parkinson’s disease mouse model compared to control mice one month after treatment. These results suggest that the astrocytes themselves, or the cells in their environment, respond to the loss of endogenous dopamine-releasing neurons by expressing factors that promote the conversion of astrocytes to iDA neurons. And Qian et al. found higher conversion efficiency in the mouse midbrain than in isolated midbrain astrocytes, indicating a role for brain derived local factors in iDA conversion. Identifying local and specific factors of damage or disease, intrinsic or extrinsic to cells, is the key to further improve the efficiency of conversion of astrocytes to neurons.
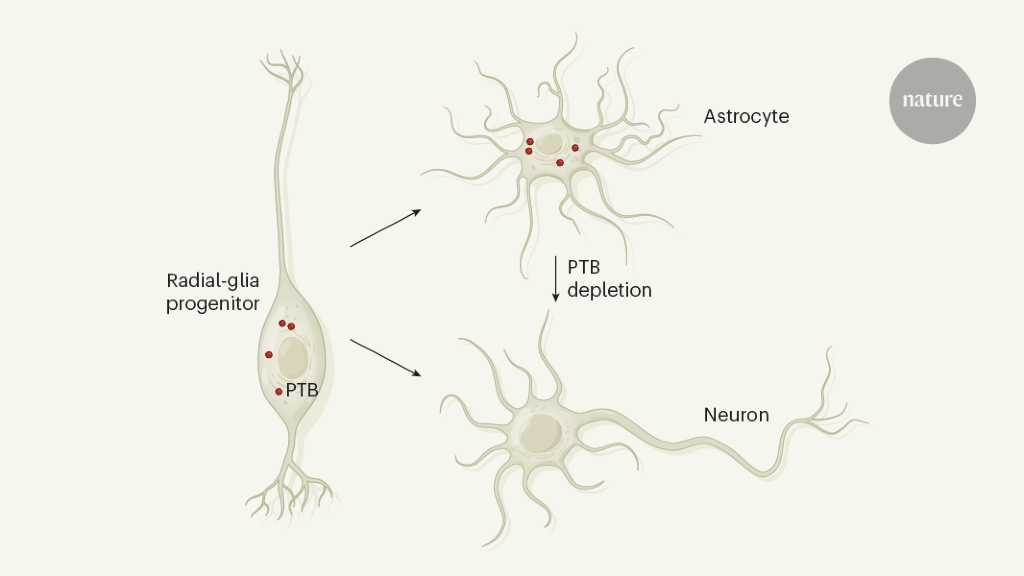
An intriguing question that arises from these studies is why astrocytes are constantly repressing neural genes. An explanation could be in the origin of the development of the cells. Astrocytes and neurons have common ancestors called radial glial progenitors – stem cell-like cells that first give rise to neurons and then differentiate into astrocytes and other glial cells that support neurons.10. In the developing midbrain of the mouse, all types of radial glial cells express Ptbp1, while differentiating precursor neurons and non-neuronseleven. Perhaps midbrain astrocytes, as descendants of the radial glia, have inherited a program to generate neurons that remain inactive unless PTB is depleted (Fig. 1). Ptbp1 is also expressed in other types of midbrain cellseleven, including endothelial and pericytic cells in blood vessels, the ependymal cells that line the ventricular cavity, and immune cells called microglia. Future studies should examine whether PTB depletion can also convert these cells into iDA neurons in animal models of Parkinson’s disease.
In order for this strategy to be useful in the clinic, it may be necessary to improve its efficiency. For example, 60–65% of infected astrocytes do not become iDA neurons. This percentage should decrease, either through more focused targeting of astrocytes in the substantia nigra, or through the introduction of factors that allow non-nigral astrocytes to become iDA neurons. It will also be important to determine the quality and authenticity of iDA cells converted at the single cell level, and to investigate whether unwanted cells are generated. Both Qian et al. and Zhou et al. provide evidence that astrocytes develop into other types of neurons, in addition to iDA cells. Furthermore, Qian et al. show that the converted iDA neurons project primarily to the septum, rather than the striatum, and that only 8% of the fibers projecting to the septum come from iDA neurons. However, on a positive note, more than half of the fibers reaching the striatum were contributed by iDA neurons. This finding, together with the demonstration that the conversion process restored striatal dopamine levels and motor activity, provides evidence of a remarkable functional reconstitution of the nigrostriatal pathway by iDA neurons.
In a final set of experiments, Qian et al. Explore one way your approach could be used in the clinic: using short nucleic acids called antisense oligonucleotides that bind to an mRNA and prevent its translation into proteins. The authors show that the local transient supply of antisense oligonucleotides against PTB led to the generation of iDA-like neurons and motor recovery in the mouse model of Parkinson’s disease, demonstrating the validity of the approach.
Future experiments should examine whether human midbrain or striatum astrocytes can also be converted to iDA, and whether the converted cell types and their targets are correct and stable for long periods. The safety of PTB depletion and the strategies used to administer treatment should also be carefully evaluated, to rule out any collateral damage to the brain cells of the host or the converted cells, or any damage resulting from the depletion of the astrocyte strategy. Although many questions remain to be answered, the simplicity and efficiency of this gene therapy approach to cell replacement makes it very attractive. Current studies promise to open a new chapter in the development of regenerative medicine for neurological disorders like Parkinson’s disease.
