[ad_1]
Saturday WHO issued a press release, which found that the organization’s Solidarity trial found no evidence that the lopinavir / ritonavir cocktail would help coronavirus patients in hospitals.

AFP / Scanpix Photo / World Health Organization (WHO)
“The study results show that mixing anti-HIV lopinavir with ritonavir did not significantly reduce the number of coronavirus deaths in hospitalized patients,” the WHO said in a statement.
The Solidarity Trials Program was established by WHO to find an effective way to combat the disease that caused the pandemic. The program evaluated five approaches to combat COVID-19: hospital care, remdesivir, hydroxychloroquine, lopinavir and ritonavir, and a mixture of lopinavir and ritonavir with interferon.
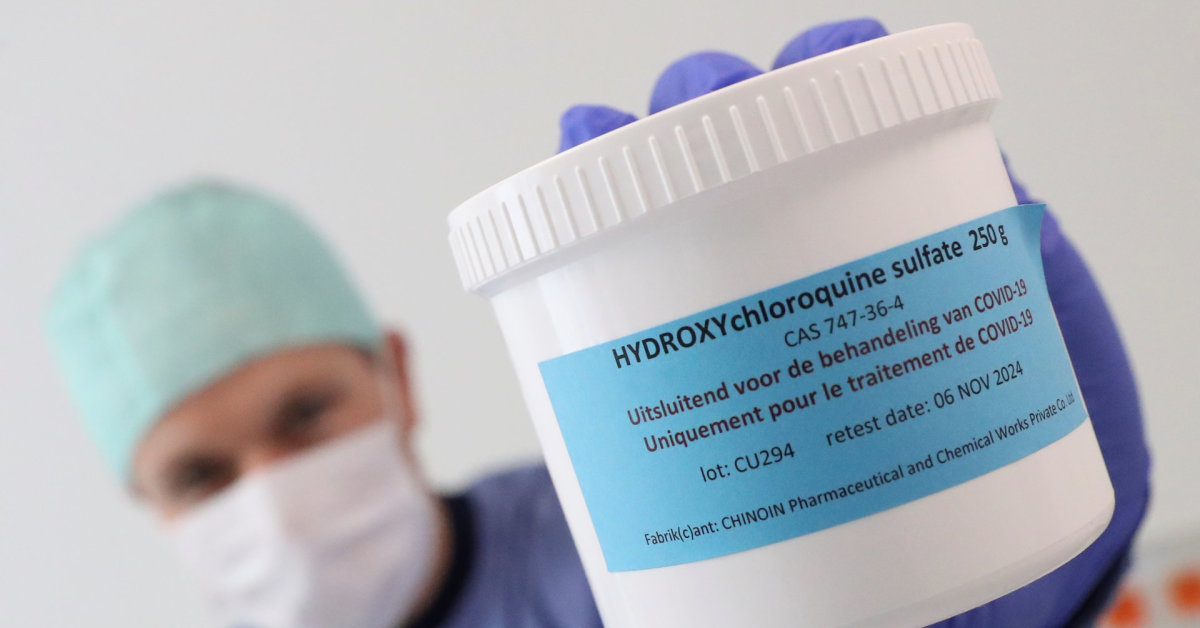
On Saturday, the organization finally confirmed that hydroxychloroquine was also removed from the test. Although there was a lack of evidence of efficacy, the antimalarial drug was effective in fighting COVID-19 repeatedly recommended by the head of the United States D. Trump.

Scanpix / SIPA Photo / Donald Short
According to the WHO, the decision to discontinue testing with these products only applies to already hospitalized patients and does not apply to studies on the effectiveness of the drug before or after COVID-19.
The European Medicines Agency (EMA) issued a recommendation in late June use the antiviral remdesivir from the WHO study to treat patients infected with the new coronavirus. On Friday The European Commission issued a formal permit on Friday use remdesivir to treat coronavirus COVID-19 infection.
Official information on COVID-19 is provided by SAM, National Center for Public Health (NVSC), European Center for Disease Prevention and Control (ECDC).
[ad_2]