[ad_1]
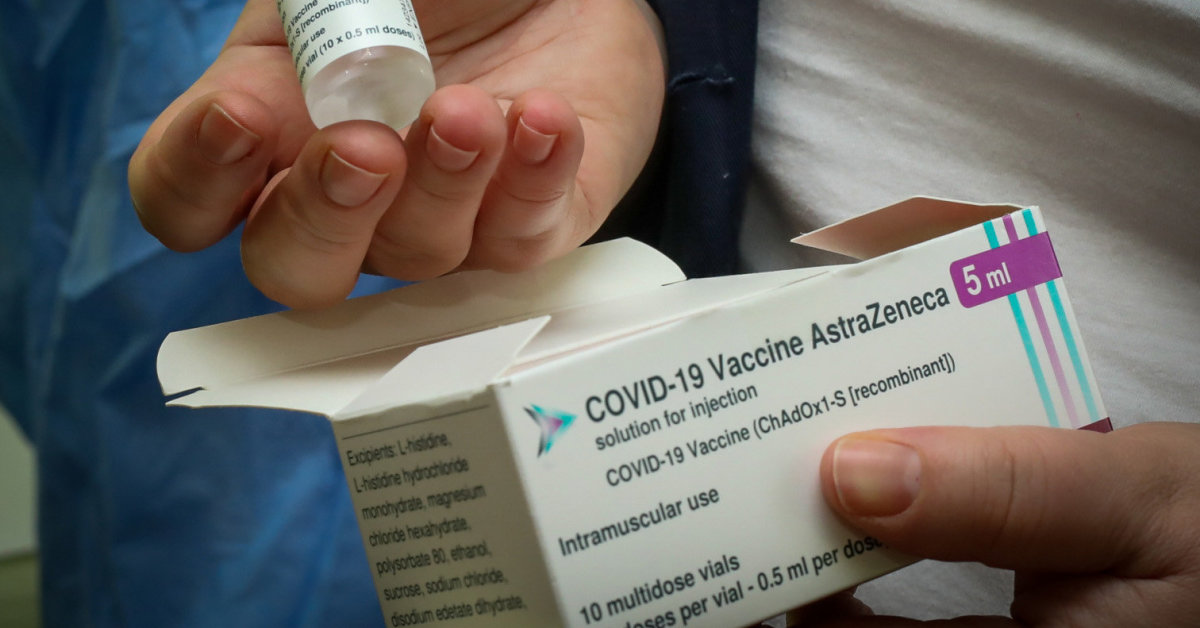
9,600 vaccines from this AstraZeneca series are reported to have been delivered to Lithuania on February 11. Currently 1932 doses are not used.
The ABV5300 series vaccines have been distributed to 17 countries in the European Union. Luxembourg, Estonia, Greece, Austria and Lithuania have temporarily suspended its use.
He died 10 days after vaccination.
According to IWT chief Gytis Andrulionis, the service received a notification on Sunday evening from the Austrian Medicines Agency that the use of an AstraZeneca vaccine against the COVID-19 series had been suspended because a recently vaccinated person had been diagnosed with multiple thrombosis.
The man died 10 days after vaccination. Another person in Austria has a pulmonary embolism, is being treated in a hospital and is recovering.
With this in mind, IWT on Monday proposed to the Ministry of Health (SAM) to temporarily suspend the use of this series of vaccines in Lithuania.
Until more information is received from the European Medicines Agency and it is found that there is really no link between previous cases of thrombosis recorded in Austria and vaccination.
Neither neighboring countries nor some other European Union countries have done the same.

Photo by Sigismund Gedvila / 15min / Gytis Andrulionis
The European Medicines Agency has also taken over the coordination and evaluation of the issue. In your opinion, there is no reason to worry. Because these serious adverse reactions and death are not associated with vaccination. However, as I already mentioned, in order to be as open as possible and avoid possible severe reactions or misinterpretations in order to protect human health, we have proposed to the Ministry to temporarily postpone those doses of the vaccine ”, said the Head of IWT.
According to G. Anddrulionis, there is no information that after the vaccines of this series have been used in our country, someone has experienced any side effects related to thrombosis.
The shipment of this series of AstraZeneca vaccines was distributed to medical institutions in Lithuania on February 15.
Reaction reports
Rugilė Pilvinienė, Senior Advisor to the ICD’s Pharmacovigilance and Poisoning Information Unit, explained that the package insert for none of the vaccines on the market, including AstraZeneca, contains information on possible effects related to thromboembolic events.
R. Pilvinienė said that in Lithuania there have been several reports of certain side effects of this series of AstraZeneca vaccine: headache, arm pain, fever, fatigue and the like.
The specialist stressed that these are reactions that are likely after vaccination with any vaccine: not only against the coronavirus, but also, for example, against influenza, tick-borne encephalitis.
As for the side effects of all the COVID-19 vaccines used in our country, according to R. Pilvinienė, there are many reports that show the so-called reactogenicity reactions.
These are severe fever, headache, bone and muscle aches. Some people have reported nausea, vomiting.
“Like many people they reported pain in their hands, which was also severe. Certain muscle disorders, changes that, to our patients’ happiness and joy, resolved in a couple of days or three and did not require more complicated methods. Simple over-the-counter medications were sufficient.
Of the most serious adverse reactions already known and reported in all countries, the so-called Belo’s palsy is likely to be mentioned, which is already included in the SmPCs of both Comirnaty and Modern. “Vaccinations, AstraZeneca has not yet done so,” said an IWT spokeswoman.
The most complex reactions to coronavirus vaccines are associated with the risk of anaphylaxis, a serious allergic reaction.
R. Pilvinienė pointed out that, although there is no information on the fact that these serious reactions in Austria are actually related to vaccination, the European Medicines Agency has not yet provided firm conclusions.
According to the IWC, this body undertakes to evaluate all reports of suspected vaccine-related adverse reactions to thromboembolic events in the European pharmacovigilance database.
Conduct research
On Tuesday afternoon, the SAM announced that the IWT, after receiving information from the Austrian competent authority, had recommended that the use of the AstraZeneca ABV5300 vaccine be discontinued.
The Austrian Federal Health Service (BASG) has received two notifications, possibly related to the vaccination of the same batch of AstraZeneca vaccine.
A study is currently underway to investigate two suspected adverse reactions in Austria, thrombosis and pulmonary embolism.
Photo by Saulius Žiūra / Skeptics
As stated in the press release, SAM and international experts emphasize that there are no objective reasons to worry or worry.
“Based on clinical trial data, thromboembolism is not a typical side effect of AstraZeneca. Initiated international analysis of side effect reports also shows no similar cases in the past. In order to ensure public safety and clear up concerns, the goal is to test any assumptions about the safety of the vaccines, ”said the SAM report.
The Ministry emphasizes that during large-scale vaccination not only in our country, but also around the world, Lithuania constantly monitors and analyzes all information on suspected adverse reactions after the COVID-19 vaccine and takes appropriate actions.
“We remind you that in mid-January the vaccination process was briefly suspended due to the use of the BioNTech and Pfizer vaccine, when SAM was informed about the possible damage to the cold chain during transport. After receiving official approval from the Pfizer representative office in Lithuania regarding the possible further use of the vaccine, the vaccination process was resumed, ”the SAM report said.
[ad_2]