
[ad_1]
According to President Gitanas Nausėda, the new delivery schedules for the COVID-19 vaccine allow 70% to vaccinate in mid-summer. Lithuanian adult population.
On Thursday, after the European Council, he announced that a new vaccine supply plan has been approved in the first and second quarters of this year.
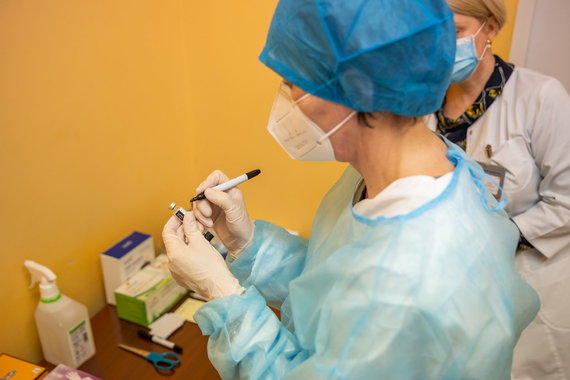
Photo by Saulius Žiūra / Vaccination of doctors from Vilnius City Clinical Hospital against COVID-19
According to him, the EU should receive 100 million in the first quarter from various producers. dose, the second another 480 million. dose of vaccine. Lithuania should have 600 thousand of them, respectively. and 3 million. dose.
“An ambitious but achievable goal”
The production and supply of vaccines to the Community was discussed on Thursday evening in a joint debate between two committees of the European Parliament (EP), the Committee on the Environment, Public Health and Food Safety (ENVI) and the Committee on Industry , Research and Energy (ITRE). meeting.
He was serviced remotely by executives from seven companies that have already developed or are still developing COVID-19 vaccines: AstraZeneca, CureVac, Johnson & Johnson, Moderna, Novavax, Pfizer and Sanofi.
At the beginning of the meeting, Stella Kyriakides, European Commissioner for Health and Food Safety, emphasized the need to vaccinate the population as soon as possible. “We have set a common target for Member States of 70%. Adults should be vaccinated before the summer. That is an ambitious target, but it can be achieved.”
As a result, he said it was important to have accurate vaccine supply forecasts and that “we must be one step ahead” when the coronavirus begins to mutate.
Reuters / Scanpix photo / Vaccine illustration
To this end, the EU has created Biological protection plan for the HERA incubator for COVID-19 varieties. Its objective is to coordinate the efforts of scientific, industrial and public institutions to promote the development of new vaccines against the virus. Within the framework of this plan, to which the EU has allocated 75 million euros. Special tests will be used for virus mutations.
However, the meeting, which lasted almost a quarter of an hour, did not focus on what to do with the SARS-CoV-2 mutations, but instead on why companies are not keeping their promises to deliver certain quantities of vaccines.
“Like a piece of soap”
Probably most of the criticism was directed towards Pascal Soriot, CEO of AstraZeneca. These days, the pharmaceutical giant reported that in the second quarter of this year The EU will only be able to deliver half of the planned vaccines. It is said to “aim to increase the productivity of the EU supply chain” and will use its “global capacity” to deliver € 180 million to the EU in the second quarter. vaccine dose ”.
The report comes in the wake of problems with the delivery of AstraZeneca and a vaccine developed by the University of Oxford in the EU in the first quarter. Even before the EU approved the vaccine in late January, the company sparked outrage among European leaders by announcing that it would not live up to its commitment to deliver $ 400 million. vaccine doses due to lack of production in European factories.
The disagreements have also sparked diplomatic tensions with Britain, which eventually withdrew from the EU, and Brussels indirectly accused AstraZeneca of favoring the country. Since the end of last year, millions of Britons have been vaccinated with the AstraZeneca vaccine alone.
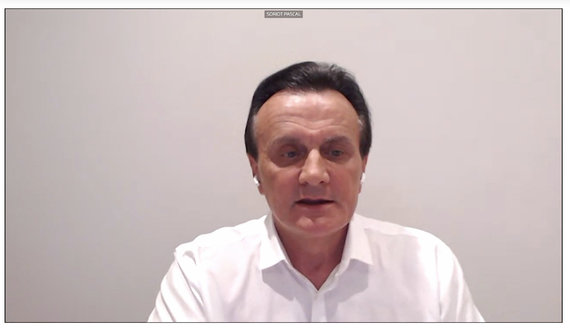
Stop shot / Pascal Soriot, CEO of AstraZeneca
Mr. Soriot, like managers at other companies, noted that it was extremely difficult not only to develop an effective vaccine in such a short time, but also to start producing it in large quantities. He called the complexity of the task unprecedented. According to him, now the company produces 100 million. doses per month, but the goal is to double this amount starting in April.
“We will aim to deliver as many doses of the vaccine as possible in the first half of the year, and we will be perhaps the largest supplier,” Soriot said, emphasizing several times that the company operated non-profit. – (…) We will endeavor to fulfill our obligations under the agreements, which is our priority ”.
The Director-General had to explain why the vaccines are being smoothly supplied to the UK, despite the fact that the EU has not only signed a contract to purchase them, but has also provided funding. The government of this country has invested a lot of money in the development of the vaccine (in the EU – in production), which is why several contracts have been signed with it. It is true that Mr Soriot assured that the vaccines produced by the reserve plant in the Netherlands would remain in the Community.
Scanpix / PA Wire / Press Association Images photo / AstraZeneca vaccine dose in preparation
“It’s very difficult for you, like a bar of soap, to catch yourself,” said Silvia Modig, a left-wing Finnish MEP. – How is it possible that you did not know your production capacity?
What happened is not a small failure, but a great failure. How can you show that we can trust that you will honor your agreements in the future and deliver on what you promised? “
According to the head of AstraZeneca, the reduction in supply is due to lower-than-expected productivity, with factories that had to produce vaccines for the EU producing less. However, even if all the British quantities were collected, the needs of the EU would not be met.
“Thousands of people are working around the clock to solve problems, secure supply, meet expectations,” Poriot explained. In his words, this is always the case when a new drug is made. Under normal conditions, manufacturers have several years to test everything, to train people. Production is now taking place in parallel with clinical trials.
Photo by Scanpix / Computer Generated Image of Coronavirus – Surface Bumps Are Famous “Needles” Against Mutating Vaccines
At the same time, the CEO of AstraZeneca took the opportunity to praise their vaccine. In Scotland, where around a million people have been vaccinated, including those over 80, 94 percent. Cases of infection were reported to have been avoided.
Efficacy is already being studied in adolescents and pregnant women
Stéphane Bancel, director of the American company Moderna, also stated that production volumes increase every week. More than 1 billion doses of the vaccine are expected to be produced next year. Ensuring that a lower dose can be effective could vaccinate 2 billion people with it. people.
He recalled that so far no one has produced vaccines based on mRNA technology. “It is not possible to ask companies to start production immediately. They have no equipment. The space needs to be redesigned ”, explained the person in charge of Moderna.
Scanpix / AP Photo / Modern test vaccine injection to patient
The process is complicated by the fact that it takes place in many places: the active substance in this company’s vaccines is produced in Switzerland, bottled in Spain and soon in France, distributed from Belgium.
Bancel also noted that the Moderna vaccine, while not as effective in protecting against the coronavirus strain found in PAR as it did in spreading from Wuhan, still helps the body produce enough antibodies to protect itself against the virus. By the way, it promises to start testing a vaccine soon to combat the so-called PAR strain of the virus.
According to Angela Hwang, president of Global Biopharma, a company belonging to the Pfizer Group, 2 billion have already been pledged this year. dose of vaccine. The company is already investigating how the vaccines it has developed affect adolescents from the age of 12 and pregnant women.
„Reuters“ / „Scanpix“ nuotr./Vakcina nuo COVID-19
Paulson Stoffels, Johnson & Johnson’s CEO and chief investigator, while the vaccine is still pending EU approval, said it is preparing to produce 200 million. dose.
“It is not enough to present a prescription”
The leaders of several vaccine development companies who attended the meeting were optimistic and predicted that they could start production in the coming months.
“We expect to receive confirmation in late May or late June,” said Franz Werner Haas, CEO of the German company CureVac. – Currently, data shows that we can expect high efficacy (the level of antibodies in the body corresponds to the condition after COVID-19 – ed. Past). We are also monitoring the situation with regard to mutations. “
He also pointed out the need to share all technology with third countries to expand production. “It is not enough to share a recipe with others, it is necessary to share the knowledge,” said the CEO of CureVac.

AFP / Scanpix photo / IRBM scientist working on a vaccine in Italy
However, it is worth the effort to produce 300 million. dose, and next year, I expected, and a billion. FWHa stressed the importance of taking into account mutations in the virus, the bar that there may be even more.
This complicates production because “it is not possible to produce vaccines against Wuhan virus and mutations in the same factory.” Finally, knowledge is needed to develop the right vaccine for them. “We hope to be able to introduce a new vaccine against all variations in 6 months,” said FW Haas.
According to the president of Novavax, Stan Erck, the vaccine developed by this American company is also effective against virus strains detected in the United Kingdom and South Africa. Efficacy against the primary virus is 94 percent, against the British strain – 86 percent.
The company also promises to produce $ 2 billion a year. doses of approximately 150 million. doses per month. “Our chances will depend on the European Medicines Agency approving the vaccine and depends on a pre-purchase agreement,” Erckas said.
Questions remain
After the meeting, the president of ENVI, Pascal Canfin from France, in the group “Renewing Europe”, summarized that listening to the explanations of the heads of the pharmaceutical companies had shed more light on the supply and production of vaccines. However, doubts remain that AstraZeneca does not appear to be able to deliver on its promises.

AFP / Scanpix Photo / Laboratory technicians prepare doses of AstraZeneca vaccine for clinical trial
“It will be problematic if it turns out in the coming weeks that AstraZeneza is not complying with the agreement,” he said.
According to the Romanian leader of ITRE, Cristiano Bușoi of the European People’s Party, the discussion was not polite and many questions were left unanswered. “We have sent a strong signal from the EP that we are monitoring the situation. And that what has been promised must be fulfilled,” he said.
[ad_2]