
[ad_1]
New data on the Moderna vaccine appeared the day after the Pfizer-developed vaccine was introduced to Americans, according to sciencealert.com
However, the Food and Drug Administration rated Moderna, which is likely the second COVID-19 vaccine approved in the United States, slightly worse than Pfizer’s variant, although Moderna’s effectiveness in protecting against the coronavirus is about the same. by 94 percent.
The vaccine, developed by Moderna, starts working in 14 days
Take a look at this table. It shows how well more than 15,000 people have been protected from COVID-19. The number of people in the study who received doses of Moderna compared to 15,000 other subjects who received placebo.
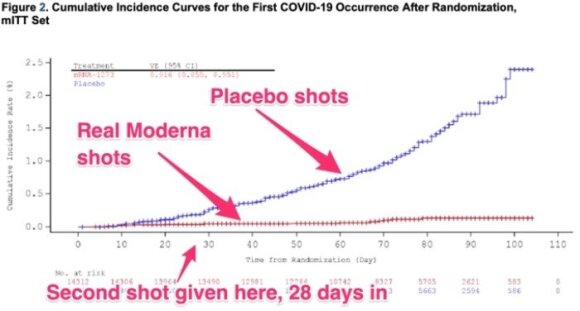
Vaccine performance compared to placebo
© FDA
Differences between Moderna and Pfizer vaccines
The vaccine efficacy diagram developed by Moderna is quite similar to the Pfizer vaccine discussed by the Food and Drug Administration last week. Like the Pfizer product, the Moderna vaccine appears to provide protection against coronavirus approximately 14 days after injection.
It is true that it is difficult to say how effective a single dose is, since almost all the people in the Moderna study after 28 days. received a second dose (subjects were also vaccinated twice in the Pfizer study; the second dose was administered 21 days later).

Vaccine performance compared to placebo
© FDA
No one in the vaccinated group developed severe COVID-19.
While waiting for the vaccine to start working properly (14 days after the second dose), only 11 people in the group vaccinated with Moderna developed COVID-19. In the control study group, 185 people were infected with the new coronavirus during the same period (approximately two months). By the way, none of the vaccinated participants developed severe COVID-19. In the control group, 30 people were seriously ill and one even died.
The side effects found in the study were mostly relatively minor, but 17 percent. participants complained of serious ailments after vaccination, such as headache, fever, and body aches. Some of them had side effects for two or three days.
In the Moderna vaccine trials, people younger than 65 were more likely to report more serious side effects. As the San Diego Union Tribune wrote, some study participants (both Moderna and Pfizer) even took a break to improve their health.
The Food and Drug Administration is also expected to approve the vaccine developed by Moderna, which will become the second coronavirus vaccine. Sources told The New York Times that permission to use the vaccine in an emergency is likely to be granted starting this Friday.
It is strictly forbidden to use the information published by DELFI on other websites, in the media or elsewhere, or to distribute our material in any way without consent, and if consent has been obtained, it is necessary to cite DELFI as the source.
[ad_2]