[ad_1]
Moderna announced its intentions to launch phase 3 clinical trials of the vaccine in July. Meanwhile, the Brazilian Institute of Bhutan announced that it has signed a clinical collaboration agreement with Sinovac Biotech and will help them conduct phase 3 clinical trials of the Chinese inactivated vaccine in humans.
According to the latest data, the new coronavirus pandemic has already claimed more than 400,000 lives worldwide. lives, totaling nearly 7.5 million. cases in 196 countries and territories. Because there is still no specific treatment for this disease or vaccine, many groups of scientists around the world are in a hurry to develop their own vaccines.
The Municipality of São Paulo (Brazil) confirmed that a contract has been signed with Sinovac from China, under which Brazil will produce the Chinese vaccine CoronaVac and the Bhutan Institute will assist in Phase 3 clinical trials of the vaccine in Brazil. The investigation will begin in July and will involve some 9,000 people in the country. If the vaccine’s effectiveness is proven, it will be produced in Brazil and should be available in the first half of 2021.
“If it works, we will be able to immunize millions of Brazilians with this vaccine,” said Joao Doria, governor of São Paulo.
In parallel, phase 2/3 clinical trials of the AZD1222 vaccine candidate, developed at the University of Oxford in Brazil, are widely accepted as one of the most promising vaccines, and has made significant progress in the vaccine career, with AstraZeneca taking significant financial risk will launch without waiting for the research results, and if the research results turn out to be good, its rollout could start as early as fall of this year.
The Chinese CoronaVac vaccine has worked very well in the preclinical phase. It occurs by growing coronavirus in a laboratory and then killing it. An article by company scientists, published in the journal Science, claims that tests on macaques have shown that the vaccine is safe and gives animals immunity against infection. Together, the Chinese company is building a commercial vaccine production line in China, which is expected to produce up to 100 million doses of CoronaVac annually.
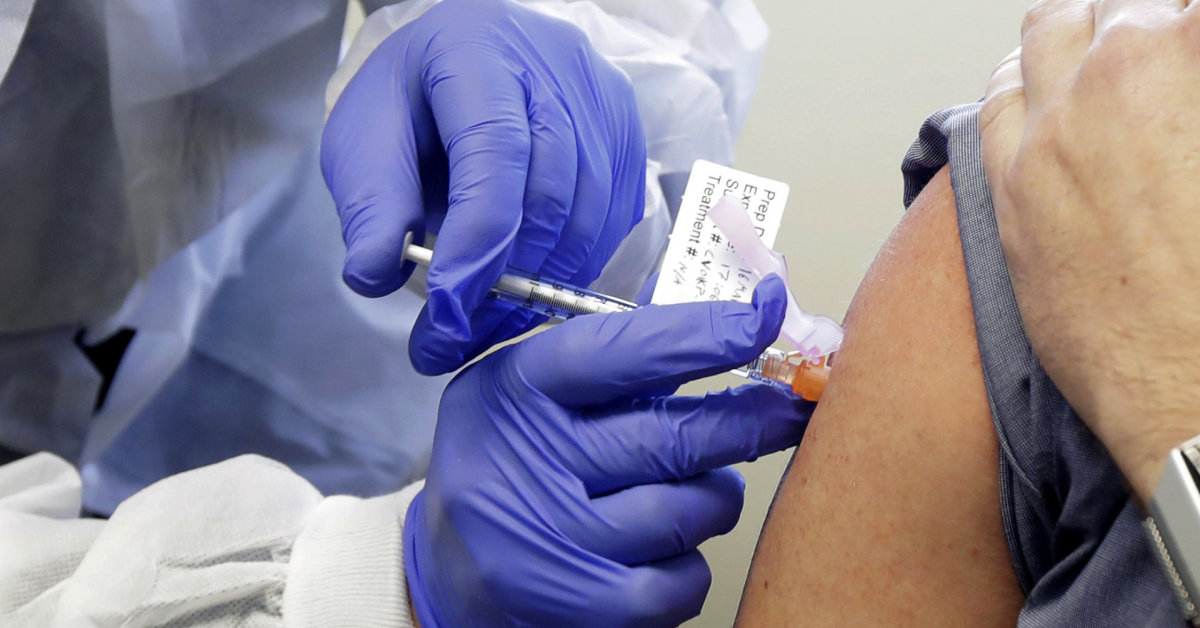
Meanwhile, the American company Moderna developed its research vaccine in collaboration with the US National Institutes of Health. USA Phase 3 clinical trials of the vaccine are scheduled to begin in the United States in July, involving 30,000 patients at a dose of 100 micrograms.
Some of the patients who volunteered to receive the study would receive the test vaccine mRNA-1273 and some would receive the control placebo. To avoid scientific error, neither patients nor doctors will know for some time whether the injected vaccine is “true” or “control.” This will allow an objective assessment of which group of patients will be most resistant (and to what extent) to the new coronavirus.
The mRNA-1273 vaccine does not contain the SARS-CoV-2 virus itself, but contains the genetic code that encodes the ‘needles’ that name the coronavirus, which cover the surface of the virus and form a corona-shaped image. With these needles, the virus recognizes proteins in human cells and attaches itself to them, then enters the interior of the cell and begins to multiply. For its part, MRNA-1273 should stimulate the body’s cells to produce a harmless conifer protein to which the human immune system will respond, thus obtaining the ability to protect the body from a true subsequent viral invasion. It is true that so-called mRNA vaccines are still completely new in medicine: they are still a new and untested technology and there are still no vaccines available on the market of this type in the world.
It should be added that neither the American nor the Chinese company have yet published the results of the phases of its previous clinical trials, in which fewer volunteers participated. Phase 1 of the clinical trials on several dozen people aims to verify any acute and severe side effects, Phase 2 involved hundreds or even thousands of people, and observed how the human immune system responds to different doses of the vaccine.
At the same time, the US National Institutes of Health. USA They hope to help developers of several more candidate coronavirus vaccines in organizing large-scale clinical trials this summer.
With the COVID-19 pandemic not only devastating human lives but also severely damaging national economies, countries around the world and their pharmaceutical companies have already started storing millions of doses of vaccines that have yet to be tested as soon as they are safe (but still not convinced). effective). For example, Operation Warp Speed, a program announced by the United States, estimates that $ 300 million should be raised by January. vaccine dose, which “cuts” even 8 months from the previously thought minimum time required for vaccine development, testing, and production.
There is no guarantee that these cumulative vaccines will be effective yet, but if efficacy is demonstrated in phase 3 clinical trials, they can be distributed very quickly.
The Vaccine Research Center of the National Institutes of Health under the direction of dr. John Mascola said at a meeting of the US National Academy of Medicine. USA On Wednesday that, if all goes well, at the end of the year it will be known which of the accumulated vaccines are effective.
It is important for researchers to test the effectiveness of vaccines that work according to different principles, thereby increasing the likelihood that at least one of the vaccines being tested is effective. The World Health Organization states that there are currently more than 120 coronavirus vaccines in various phases of research.
[ad_2]